Atomic Theory 1
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
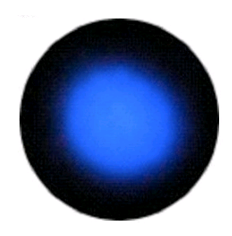
S-Orbital
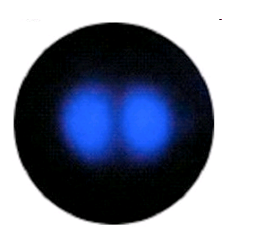
P-orbital
What does the Greek model say the universe is composed of?
The atoms and the void in which they exist and move
What does Dalton’s model say all matter is made of?
Atoms
What 3 things does Dalton’s Model say that atoms are?
Indivisible
indestructible
Identical in mass and properties
What does Dalton’s model say that compounds are formed by?
A combination of 2 or more different kinds of atoms
What does Dalton’s Model say a chemical reaction is?
A rearrangement of atoms
What does the Thomson’s Model say atoms are?
Uniform spheres of positively charged matter in which electrons are embedded
What does Rutherford’s Model say about the size of negatively charged electrons?
They’re small
What does Rutherford’s Model say that negatively charged electrons do?
Orbit around the nucleus like in a small scale cosmic model
What does Bohr’s Model say electrons do?
Rotate around the nucleus in orbits that have a set size and energy
Where does Bohr’s Model say that the lowest energy is found?
In the smallest orbit
What does Bohr’s Model say the energy of the orbit is related to?
Its size
When does Bohr’s Model say radiation is absorbed or emitted?
When an electron moves from one orbit to another
What is the Heinsenberg’s Uncertainty Principle?
Orbitals are regions where electrons are likely to be found
What is the wavelength of a moving body inversely proportional to?
Its momentum
How do you calculate momentum?
Mass (kg) x velocity (m/s)
What is the motion of large bodies better described by?
Classical mechanics
What is the motion of particles better described by?
Quantum mechanics
Long wavelength has?
Small frequency and energy
Short wavelength has?
High frequency and energy