Inorganic Chemistry Exam Style Qs
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
A student wants to carry out a titration experiment.
State why universal indicator is not a good choice for this experiment and suggest an indicator that would be more suitable (2)
m1: colour change is gradual, not sharp or defined m2: methyl orange/phenolphthalein/litmus
A student uses the precipitation method to prepare lead(II) bromide.
The equation for the reaction she uses is
Pb(NO3 )2 (aq) + 2NaBr(aq) → PbBr2 (s) + 2NaNO3 (aq)
Describe how she could use solutions of lead(II) nitrate and sodium bromide to obtain a pure, dry sample of lead(II) bromide (5)
m1: mix/add the two solutions m2: together m3: stir m4: filter m5: wash with water m6: dry in oven
2Br- + 2e- → 2Br
Identify the two mistakes in her half-equation (2)
m1: electrons should be on the left side m2: 2Br should be Br2
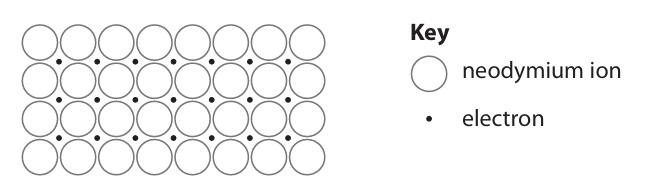
Explain, with reference to the diagram, why neodymium is malleable and a good conductor of electricity. (4)
m1: neodymium ions in layers/rows m2: slide/slip over each other m3: delocalised electrons m4: are free to move
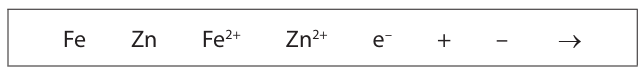
If the layer of zinc on the nail is scratched, sacrificial protection prevents the iron from rusting.
Explain, with the help of two ionic half-equations, how this type of sacrificial protection works.
Use symbols from the box in your equations. You may use each symbol once, more than once or not at all. (4)
m1: zinc more reactive than ion m2: better reducing agent than ion m3: zinc corrodes instead of iron m4: Zn → Zn2+ + 2e occurs m5: Fe → Fe2+ + 2e doesn’t occur
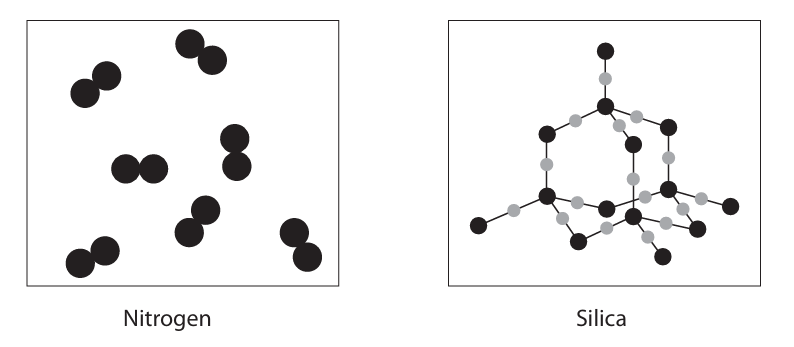
The main substance that acts as an insulator in this method of transmission of electricity is air, which is mostly nitrogen.
The power lines are supported by solid insulators. Most solid insulators are manufactured using silica.
The diagram shows the structures of nitrogen and silica
Explain, in terms of bonding and structure, why nitrogen is a gas at room temperature but silica is a solid with a high melting point (5)
NITROGEN
m1: simple molecular structure, m2: intermolecular forces of attraction m3: easily overcome
SILICA
m1: giant structure m2: covalent bonds m3: need a lot of energy to break

The Taj Mahal is a famous building in India. It is made out of a form of calcium carbonate called marble
The appearance of the marble has changed gradually over the years because of the effects of sulfur dioxide in the atmosphere.
Describe how sulfur dioxide has caused this change in appearance. (3)
m1: dissolves in/reacts with (rain) water
m2: to form an acidic solution/acid rain
m3: which reacts with/corrodes the marble/calcium carbonate
State two similarities and two differences between the reactions of lithium and potassium with water. (4)
SIMILARITIES:
m1: floats/moves around
m2: effervescence
DIFFERENCES:
m1: potassium forms a ball
m2: potassium produces a flame
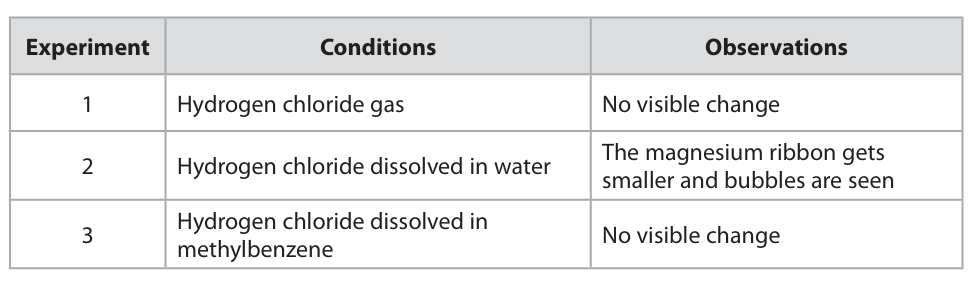
Silver nitrate solution and dilute nitric acid are added to the solution produced in experiment 2.
State what is observed and name the substance responsible for this observation. Explain why dilute nitric acid is added. (3)
m1: white precipitate m2: silver chloride m3: to prevent other precipitates from forming
The student then mixed the volumes of aqueous ammonia and phosphoric acid found in the titration.
Describe how to use the method of crystallisation to obtain a pure dry sample of the salt from this mixture. (3)
m1: heat/boil until crystals start to form m2: leave solution to cool m3: filter to obtain crystals
The order of reactivity of three metals is
most reactive
zinc
iron
tin
least reactive
Iron objects can be prevented from rusting by coating them with zinc or tin. Some of these objects may be scratched when used, so the coating may come off.
Use the order of reactivity of the metals to suggest why coating these objects with zinc is more effective than coating them with tin (3)
M1 zinc corrodes/reacts instead of iron
M2 iron corrodes/reacts instead of tin
M3 correct reference to order of reactivity of all three metals
Lead(II) chloride is an insoluble salt that can be prepared by reacting lead(II) nitrate with sodium chloride.
Describe how you would prepare a pure, dry sample of lead(II) chloride starting from solid lead(II) nitrate and solid sodium chloride (5)
m1: dissolve both lead nitrate and sodium chloride in water m2: add the two solutions m3: filter m4: wash residue m5: dry on filter paper
Outline how you would produce a pure, dry sample of lead(II) sulfate from the reaction mixture in (3)
m1: filter m2: wash/rinse residue m3: place in warm oven
Describe a method the student should use to find the accurate volume of sodium hydrogen sulfate solution needed to neutralise the 25.0cm3 of sodium hydroxide solution (5)
m1: use pipette to add 25cm3 of alkali to clean conical flask
m2: add a few drops of indicator and put conical flask on white tile
m3: fill burette with acid and note starting volume → read meniscus at eye level
m4: slowly add acid from burette to alkali in conical flask, swirling mix
m5: stop adding when indicator changes colour
m6: note final volume reading and calculate how much acid you added in total
m7: repeat until concordant results reached and calculate mean volume required to neutralise alkali
The student forms a solution of sodium sulfate after completing the titration
Describe how the student can obtain pure dry crystals of hydrates sodium sulfate from the solution (4)
m1: filter
m2: heat/boil solution
m3: to evaporate some water
m4: leave to crystallise
m5: pour of excess liquid
m6: dry in warm oven
Explain why potassium is more reactive than lithium.
Refer to atomic structure in your answer (3)
m1: more electron shells
m2: outer electron shell is further from nucleus
m3: weaker attraction m4: likely to react and form ions