C+M Exam 1
0.0(0)
0.0(0)
Card Sorting
1/159
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
160 Terms
1
New cards
2
New cards
Most mutations are _, some are _ and some can contribute to _
silent, harmful, increased ability of the organism to survive and adapt
3
New cards
all diseases are__
disturbances at the cellular level
4
New cards
as ions become hydrated, the attractive force between them is _, and the charged species dissolves in the water
reduced
5
New cards
hydrophobic effect
nonpolar substances are EXCLUDED from the solvation netweork
6
New cards
ordered water cage
is around each nonpolar molecule, limits water molecules from interacting with other water molecules
7
New cards
lowest energy state in hydrophobic effect
has maximum water-water hydrogen bonding and minimal exposure of hydrophobic surfaces
8
New cards
gene
region of DNA that can be expressed to produce a final functional product that is either a protein or RNA molecule
9
New cards
proteins add___
functionality
10
New cards
functionality
puts cell’s genetic info into ACTION
11
New cards
functions of proteins
1. structural
2. movement
3. defense
4. regulation
5. transport
6. catalysis
12
New cards
each protein normally folds into a __ stable conformation
single stable
13
New cards
general formula of an amino acid
1. amino group
2. carboxyl group
3. side chain group (R)
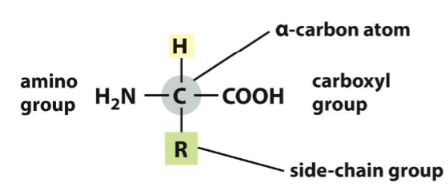
14
New cards
how many side chains are there?
20
15
New cards
At pH __ both the amino and carboxyl group are ionized
7
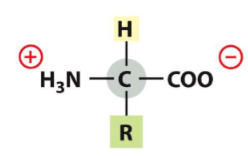
16
New cards
nonpolar side chains
some can be remembered with “glaciers in alaska valiently locate isolated prowlers”
1. gycline (G)
2. alanine (A)
3. valine (V)
4. leucine (L)
5. isoleucine (I)
6. proline (P)
Then there are
1. Phenylalanine (F)
2. Methionine (M)
3. Tryptophan (W)
4. Cysteine (C)
1. gycline (G)
2. alanine (A)
3. valine (V)
4. leucine (L)
5. isoleucine (I)
6. proline (P)
Then there are
1. Phenylalanine (F)
2. Methionine (M)
3. Tryptophan (W)
4. Cysteine (C)
17
New cards
Uncharged polar amino acids
Asparagus Never Gives Quacks Seriously Sufficient To Thoroughly Tyre You.
1. Asparagine (N)
2. Glutamine (Q)
3. Serine (S)
4. Threonine (T)
5. Tyrosine (Y)
1. Asparagine (N)
2. Glutamine (Q)
3. Serine (S)
4. Threonine (T)
5. Tyrosine (Y)
18
New cards
Positive (basic) polar amino acids
Can be remembered with “basically, His Lost kid Always returned”
1. histidine (H)
2. lysine (K)
3. Arginine (R)
1. histidine (H)
2. lysine (K)
3. Arginine (R)
19
New cards
Negative (acidic) polar amino acids
As peter digested the glue, his stomach became acidic
1. Aspartic acid (D)
2. Glutamic Acid (E)
1. Aspartic acid (D)
2. Glutamic Acid (E)
20
New cards
chiral carbons exist when the alpha carbon is attached to __ different groups
4
21
New cards
molecules with chiral carbons exist as __
stereoisomers
22
New cards
L-amino acids form a ___ helix
right handed
23
New cards
(T/F) Enzymes are chiral
T
24
New cards
Protein structure dictates __
function
25
New cards
peptide bonds
amide linkage that joins the amino acids
26
New cards
primary level of protein organization
linear amino acid sequence
27
New cards
secondary level of protein organization
basic folding units (alpha helices and beta pleated sheets)
28
New cards
tertiary structure of protein organization
3-D structure of a folded protein - non-covalent interactions of side groups
29
New cards
quaternary structure of protein organization
complete 3D structure of proteins that contain more than one polypeptide chain
30
New cards
Sequences are listed from _ terminus to _ terminus
N to C
31
New cards
a protein’s 3D structure is determined by the
order of amino acids in its chains
32
New cards
backbone of the polypeptide is composed of
everything but the R group
33
New cards
alpha helix
1. occurs when there is H bonding between N-H and C=O groups in polypeptide backbone
2. R groups project AWAY from helix
3. can be amphipathic - both polar and nonpolar (happens when 1 side is polar and the other side is nonpolar)
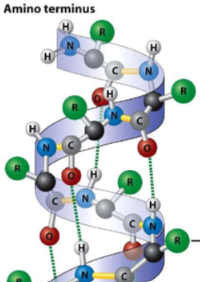
34
New cards
coiled coil
* supersecondary structure from multiple alpha helixes wrapping around one another
* 2+ helices have most of their nonpolar side chains on 1 side, twist around each other with side chains facing inwards (hydrophobic effect)
* 2+ helices have most of their nonpolar side chains on 1 side, twist around each other with side chains facing inwards (hydrophobic effect)
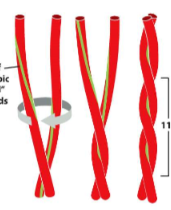
35
New cards
beta pleated sheet
* forms when 2+ polypeptide side chains line up side by side
* each beta strand is fully extended and stabilized by H bonding between N-H and carbonyl groups
* Can be parallel or antiparallel
* each beta strand is fully extended and stabilized by H bonding between N-H and carbonyl groups
* Can be parallel or antiparallel
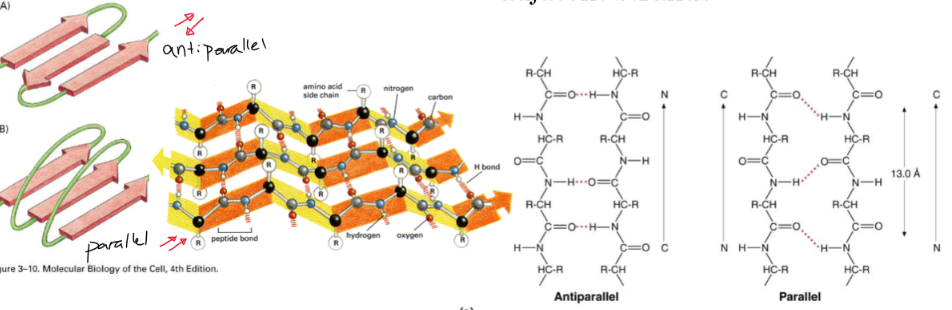
36
New cards
example of beta pleated sheet strength in real life
spider silk
37
New cards
protein domain
* a substructure produced by any contiguous part of a polypeptide that can fold independently into a stable, compact structure
* typically consist of 50-350 amino acids
* each domain conders a specific function to the protein
* even a tertiary structure can have domains
* typically consist of 50-350 amino acids
* each domain conders a specific function to the protein
* even a tertiary structure can have domains
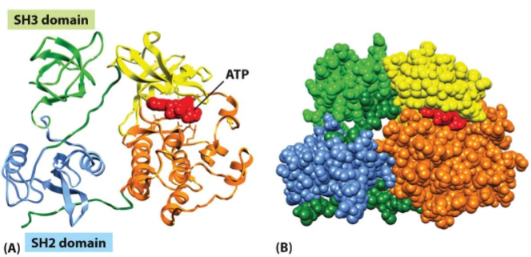
38
New cards
globular proteins vs. fibrous proteins
globular - dynamic and soluble in water
fibrous - insoluble in water, structural, elongated
fibrous - insoluble in water, structural, elongated
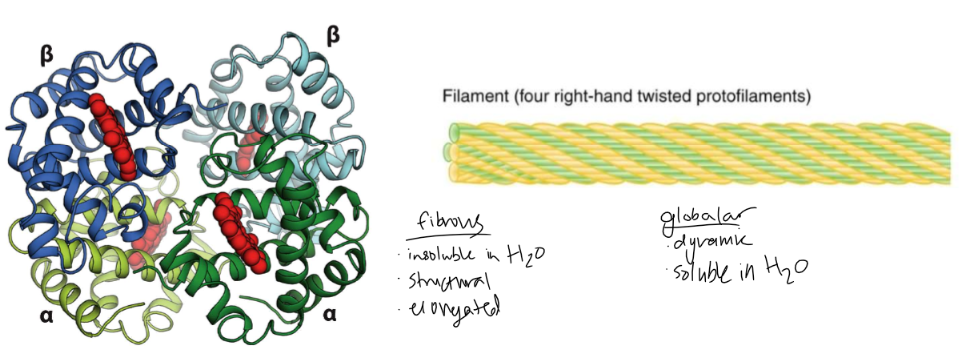
39
New cards
non-covalent interactions that stabilize tertiary structure
1. van der waals attractions: weak bond froms at a close range between nonpolar atoms
2. hydrogen bonding (weak on its own but can have multiple H-bond that make it strong) - hydrogen atoms and usually O or N
3. Electrostatic attractions - salt bridges (attraction between ionic groups of opposite charge)
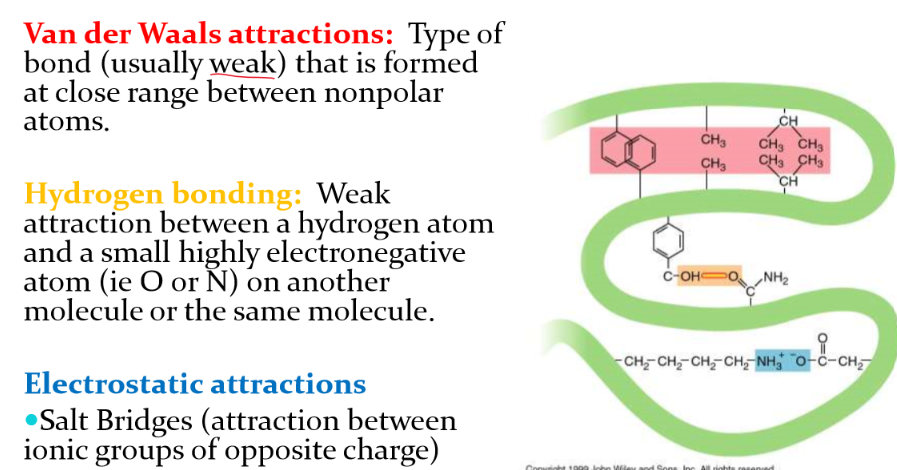
40
New cards
many inheritable genetic disorders are due to ___
deficiency/absence/excess activity of one or more enzymes
41
New cards
how can measurements of enzyme activity be useful?
diagnostics
42
New cards
many drugs exert their biological effects through interactions with __
enzymes
43
New cards
enzymes __ activation energy
lower
44
New cards
(T/F) Enzymes alter the standard free energy of a reaction
FALSE
45
New cards
phosphotases
catalyze hydrolytic removal of a phosphate group from a molecule (remove phosphate groups)
46
New cards
kinases
catalyze the addition of a phosphate group to a molecule (add phosphate groups)
47
New cards
ATPase
hydrolyze ATP. energy-harnessing ATPase activity as part of their function (nucleotide regulation)
48
New cards
GTPase
hydrolyze GTP, help regulate cell processes (molecular switch - either active or inactive) (nucleotide regulation)
49
New cards
protease
break down proteins by hydrolyzing peptide bonds between amino acids
50
New cards
(T/F) the active site uses only one amino acid side chain to coordinate making/breaking bonds with substrates
FALSE - SEVERAL AMINO ACID SIDE CHAINS INVOLVED
51
New cards
What is the 3D structure of the reactive center formed by?
folded domains of the protein
52
New cards
enzymes are ___ for their substrates
highly specific
53
New cards
(T/F) Enzymes are consumed
FALSE - can be used over and over again
54
New cards
substrate
reactant that binds to the active site of a protein
55
New cards
induced fit model
the shape of the active sit becomes truly complementary only after the substrate begins binding to the enzyme
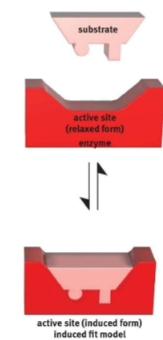
56
New cards
proteins can have slight conformational changes
gives them flexibility - is how induced-fit model can happen
57
New cards
ligand induced conformational changes
increased or decreased binding affinity of an enzyme from a DIFFERENT MOLECULE
Ex. After glucose binds to hexokinase, there is a 50 fold increase in the affinity of the enzyme for ATP
Ex. After glucose binds to hexokinase, there is a 50 fold increase in the affinity of the enzyme for ATP
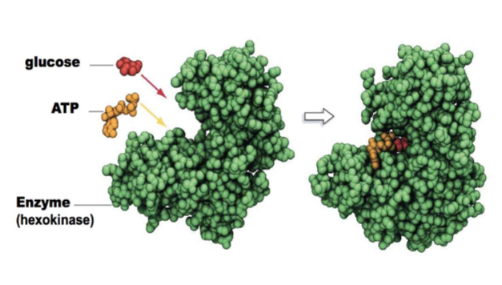
58
New cards
general strategies of enzyme catalysis
1. align/position reactants
2. induce charge state
3. deform reactants
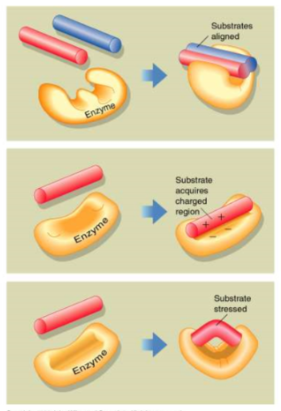
59
New cards
algin/position reactants
* fewer conformations to explore
* increase efficiency
* increase efficiency
60
New cards
induce charge state
amino acids in active site interact with reactants via charge, polarity
61
New cards
deform reactants
* enzyme strains substrate
* forcing a transition state, favor reaction
* forcing a transition state, favor reaction
62
New cards
lysozyme
* catalyzes the cutting of polysaccharide (sugar) chains in cell walls of bacteria
* catalyzes a hydrolysis reaction: adds water to bond between two adjacent sugar groups in sugar chain to cause bond breakage, reaction is favorable
* catalyzes a hydrolysis reaction: adds water to bond between two adjacent sugar groups in sugar chain to cause bond breakage, reaction is favorable
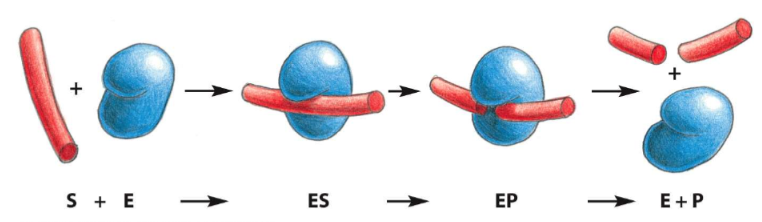
63
New cards
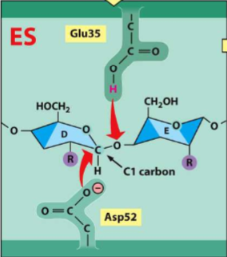
lysozyme reaction pathway
1. sugar D is forced into a strained conformation
2. Gly35 serves as an acid to donate a H+ to sugar E
3. Asp52 attacks C1 of Sugar D, TRANSIENT COVALENT BONDS form between Asp and Sugar D
4. Hydrolysis of glycosidic bond (sugar-sugar bond)
5. Glu35 polarizes the water molecule, oxygen attacks C1
6. COVALENT BOND BROKEN between Asp and C1 (sugar D)
7. Lysozyme returned to initial state (EP formation)
review the slide for this - definitely on exam
64
New cards
Enzyme characteristics
1. enzymes are catalysts - lower activation energy, promote the transition state
2. Enzymes are highly specific to reaction they catalyze
3. Enzymes work in moderate temperature
4. Enzymes are NOT consumed in reactions
5. Enzymes CAN be regulated
65
New cards
types of protein regulation
1. allosteric regulation
2. irreversible inhibition
3. post translational modifications
4. proteolytic processing
1. nucleotide regulation
66
New cards
Most proteins are allosteric, which means that ___
binding at one of the sires causes a shift from one folded shape to a slightly different folded shape
67
New cards
allosteric inhibition
binding of one molecule inhibits binding of a substrate (can’t both be binded)
68
New cards
allosteric activation
binding of one molecule allows for the binding of a substrate (both want the same conformation)
69
New cards
feedback inhibition (negative feedback)
product produced late in the reaction pathway inhibits an enzyme that acts earlier in the pathway
70
New cards
Example of allosteric activation
ADP → ATP
71
New cards
ADP is an indicator of __
low energy status in the cell
72
New cards
cooperative allosteric transition
multiple binding sites where binding at one site increases the affinity for binding in the remaining sites (4 oxygen can bind to one hemoglobin)
73
New cards
example of irreversible inhibitor
aspirin
74
New cards
reversible phosphorylation/dephosphorylation
* can activate OR inactive an enzyme
* can mask OR create a binding site
* Ex. cyclin-Cdk complexes of cell-cycle control system
* can mask OR create a binding site
* Ex. cyclin-Cdk complexes of cell-cycle control system
75
New cards
proteolytic processing
some proteins/enzymes are made in inactive forms and then become active after proteolytic processing and cleavage of the precursors
Ex. insulin production
Ex. insulin production
76
New cards
ATP
energy currency of the cell
77
New cards
enzymes that couple energy transduction to mechanical work
nucleotide binding and hydrolysis function in a variety of enzymes to perform work
78
New cards
different classes of mechanical enzymes
1. membrane transporters
2. molecular motors/machines (ATPases)
79
New cards
GTPases function as molecular clocks
Ras Protein (GTPase) plays a role in cell signaling
80
New cards
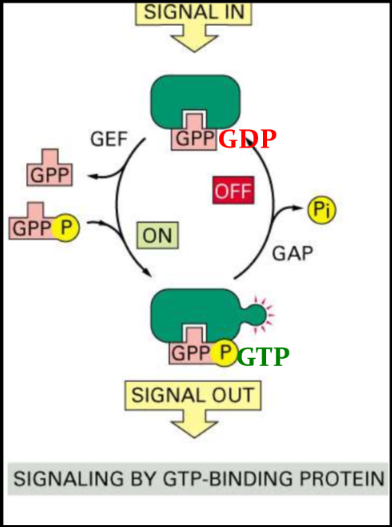
GTPase regulation
* GTPase is an indirect type of activation
* GAP - GTPase activating protein - does NOT activate a protein but it hydrolyzes GTP to turn off GTPase
* GEF - guanine exchange factor - promotes dissociation of GDP
* GTP bound = active
* GDP bound = inactive
* GAP - GTPase activating protein - does NOT activate a protein but it hydrolyzes GTP to turn off GTPase
* GEF - guanine exchange factor - promotes dissociation of GDP
* GTP bound = active
* GDP bound = inactive
81
New cards
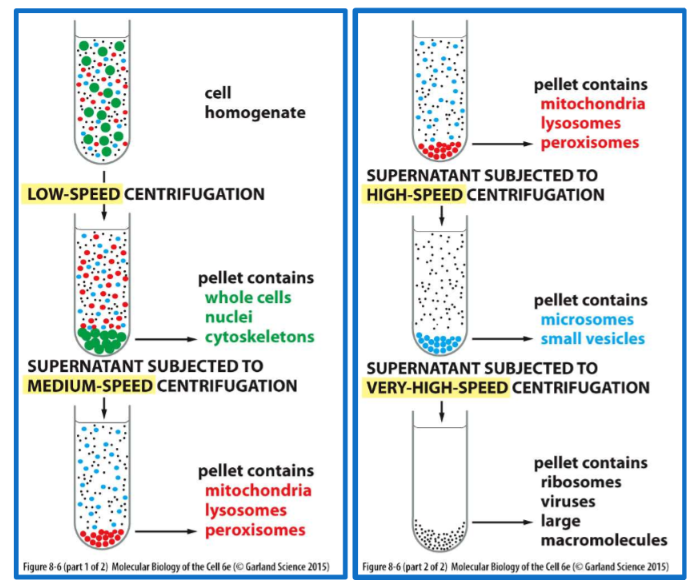
cell fractionation
* separating cells into their component fractions
* done through osmotic shock, ultrasound vibration, forced through a small orifice, blender
* plasma membrane and ER membrane retain biochemical properties
* most organelles are left intact
* done through osmotic shock, ultrasound vibration, forced through a small orifice, blender
* plasma membrane and ER membrane retain biochemical properties
* most organelles are left intact
82
New cards
homogenate (or extract)
suspension of cells, contains organelles with a distinct size, charge, and density
83
New cards
how do you purify mitochondria from cell homogenate?
centrifuge at a speed LOWER than what is required to pellet mitochondria
84
New cards
supernatant
poured into a new tube and centrifuged at a rate to cause mitochondria to pellet
85
New cards
salting out
* using salt to purify proteins through water interactions
* increases protein-protein interactions
* every protein has a characteristic salting out point
* salt can be denaturing so which salt you choose matters
* increases protein-protein interactions
* every protein has a characteristic salting out point
* salt can be denaturing so which salt you choose matters
86
New cards
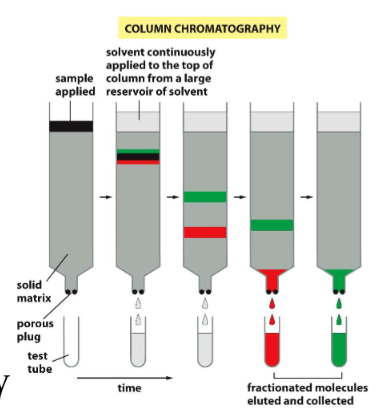
chromatography
* mixture of proteins in solution pass through a column containing a porous solid matrix
87
New cards
what is the mobile phase in chomatography?
mixture of proteins
88
New cards
what is the stationary phase in chromatography?
the column and everything in it (resin beads, porous solid matrix,..)
89
New cards
how do proteins slow down in chromatography?
based on interactions with the matrix, separated as they flow through the column
90
New cards
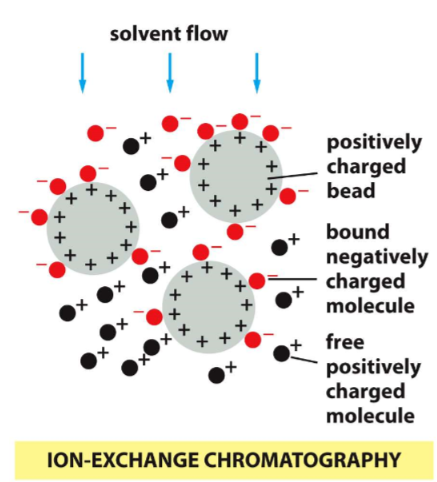
ion-exchange chromatography
proteins are separated according to chargei
91
New cards
isoelectric point (pI)
pH when molecule has no charge
92
New cards
cation exchange resins
bind to positive chargesa
93
New cards
anion exchange resins
bind to negative charges
94
New cards
elution
process of extracting a substance that is absorbed by another by washing it with a solvent
95
New cards
pH affects the net charge of a protein
* in solutions more basic than the pI, the amino group donates a proton
* in solutions more acidic than the pI, the carboxyl group accepts a proton
* in solutions more acidic than the pI, the carboxyl group accepts a proton
96
New cards
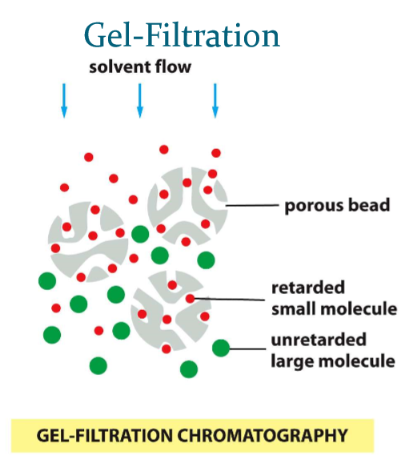
Gel-filtration chromatography
aka “size exclusion”
* molecules that are small will linger in porous beads
* larger molecules elute faster
* molecules that are small will linger in porous beads
* larger molecules elute faster
97
New cards
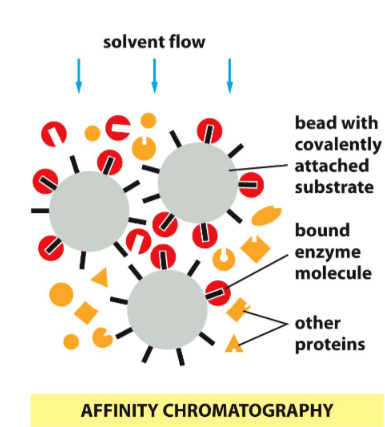
affinity chromatography
* uses non-covalent bonding affinity between protein and ligand
* ligand is covalently bound to insoluble matrix
* Ex. bound substrate (affinity for enzyme), bound antibody (affinity for specific protein)
* ligand is covalently bound to insoluble matrix
* Ex. bound substrate (affinity for enzyme), bound antibody (affinity for specific protein)
98
New cards
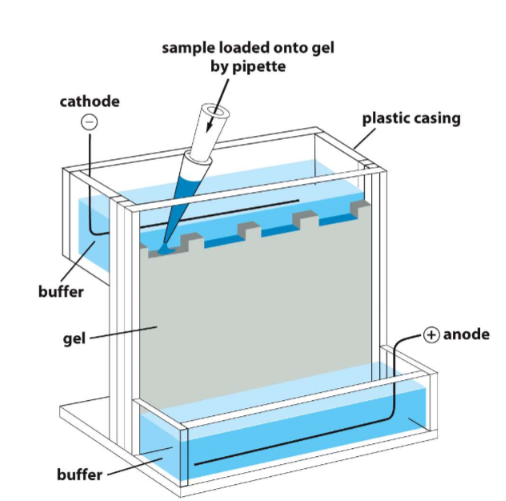
SDS-PAGE
* proteins usually have a net charge
* electric field is applied to solution with protein molecules
* protein migrates at a rate that depends on its net charge, size, and shape
* electric field is applied to solution with protein molecules
* protein migrates at a rate that depends on its net charge, size, and shape
99
New cards
metabolic flexibility
quick changes in concentrations of key regulatory enzymes, hormones, receptors, etc.
100
New cards
chaperones function
1. assist
2. rescue
3. protect