5 Electrons and bonding Checklist
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
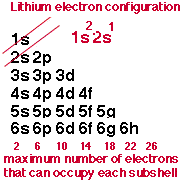
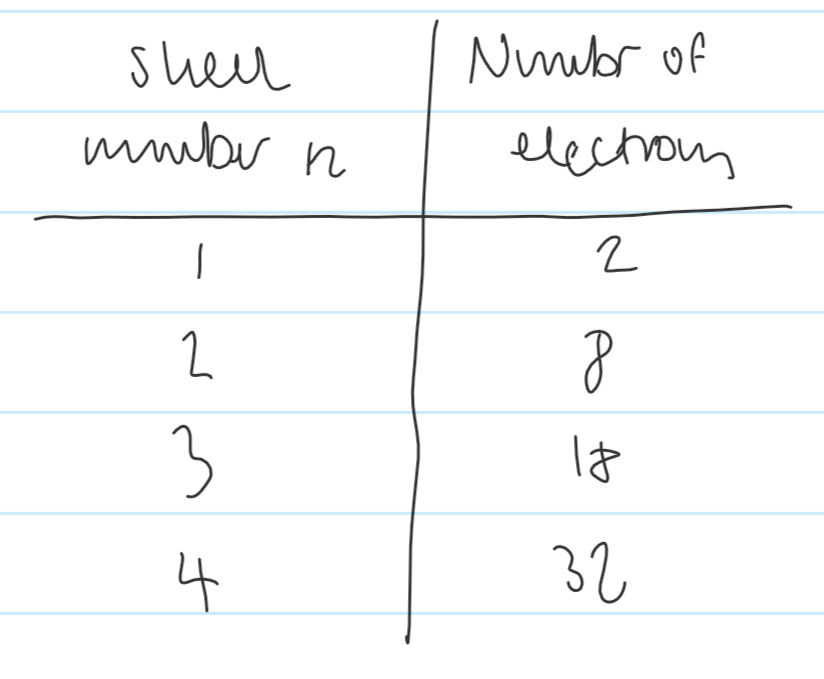
Give the number of electrons that can fill the first four shells
Describe atomic orbitals
The region around the nucleus that can hold up to two electrons with opposite spins
What is the shape of a s-orbital
Spherical
What is the shape of a p-orbital
Dumbbell shaped
rules of orbitals
orbitals fill in order of increasing energy
electrons pair with opposite spins
orbitals with the same energy are occupied first
What is within a shell, and what is within that?
Within a shell, there are subshells
Within subshells there are orbitals
What is the electron configuration of Hydrogen
1s1
What is the electron configuration of Helium
1s2
What is the electron configuration of Neon
1s22s22p6
Total number of electrons is 10
What is the electron configuration of Argon
1s22s22p63s² 3p⁶
Total number of electrons is 18
How many electrons can the second shell hold up to?
8 electrons
2s2, 2p6
How many orbitals in the second shell
4 orbitals
1 s-orbital, 3 p-orbitals
How many electrons can the third shell hold up to
18 electrons
3s2, 3p6, 3d10
How many orbitals in the third shell
9 orbitals
1 s-orbital, 3 p-orbitals, 5 d-orbitals
How many electrons can the fourth shell hold up to
32
4s2, 4p6, 4d10, 4f14
How many orbitals in the fourth shell
16 orbitals
1 s-orbital, 3 p-orbitals, 5 d-orbitals, 7 f-orbitals
Why do some subshells overlap
The further from the nucleus, the closer together the energy levels become
When using the periodic table for electronic configuration, what does the period show?
what number the highest energy subshell will be
e.g. in the 3rd period of the p-block, it will be a 3p subshell
When using the periodic table for electronic configuration, what does the column of the block show?
how many electrons in the highest energy subshell
e.g. in the 5th column of the p-block, p^5
def ionic bonding
the strong electrostatic attraction between positive and negative ions
def isoelectronic
to have the same electronic configuration as a noble gas when ions form
def ionic lattice
in an ionic compound, millions of ions are packed together in a regular cubic arrangement, joined by ionic bonds. this forms a giant 3d structure called an ionic lattice
the ionic lattice will continue to build this way until there are no more ions left to add
the structure of the ionic lattice affects the properties of the ionic compound
what 2 processes does solubility require in ionic substances
the ionic lattice must be broken down
water molecules must attract and surround the ions
what does the solubility of an ionic compound in water depend on
charge and size - bigger ions have more electron shielding so nuclear attraction is less
the relative strength of the attractions within the giant ionic lattice of the attraction between ions and the attractions between ions and water molecules
solubility decreases as ionic charge increases
with ionic compounds that have large ionic charges the attraction in the giant ionic lattice have a greater effect than the attraction of the polar water molecules
when is something soluble
if the energy released upon hydration is the same as the energy required to break the giant ionic lattice
def dissolve
when water molecules surround small components of what was put in the water
why are ionic compounds soluble in water
because of their charge. water is a polar solvent and the polar water molecules break down the lattice and surround each ion in solution
when is something dissolved
when the ion is surrounded in solution/ solvated
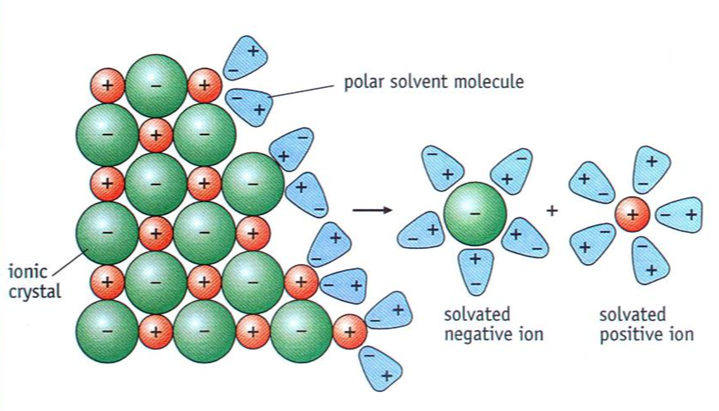
what is the structure of an ionic compound
crystalline because of the regular cubic shaped giant ionic lattice
why are ionic compounds brittle
because of their giant ionic lattice, if one layer is shifted then like ions will be over each other therefore that layer will be repelled
what is a coordination number
the number of oppositely charged ions that can surround the ion in the middle
shown in a ratio e.g. Na has a coordination no. of 6:6
def covalent bond
an electrostatic attraction between a pair of shared valence electrons and the nuclei of the atoms
or
the overlap of atomic orbitals, each containing one electron, to give a shared pair of electrons
difference between covalent vs ionic bonds
covalent:
attraction is localised, activity is solely between the shared pair of electrons and the nuclei of the two bonded atoms
covalent substances can exist as molecules
what is the octet rule
all atoms in a molecule have to have 8 valence electrons to become stable. in a covalent bond, atoms share their electrons with each other to satisfy the octet rule
(There are exceptions)
What is the strength of a covalent bond
the strength of a covalent bond is shown by its average bond enthalpy (measures the energy required to break a covalent bond). The stronger a bond is, the more energy is required to break it, and so the greater the value of the average bond enthalpy
def dative covalent bond
a covalent bond in which the shared pair of electrons has been supplied by one of the bonding atoms only. in a dative covalent bond the shared electron pair was originally a lone pair of electrons on one of the bonded atoms.
e.g. a when an ammonia molecule donates its lone pair of electrons to a H+ ion (H+ ions has no electrons)
electron configuration for chromium Cr
1s2 2s2 2p6 3s2 3p6 3d5 4s1
electron configuration for copper Cu
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
subshell triangle thing