module 6 - organic chemistry & analysis
1/124
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
125 Terms
What are the characteristics of benzene?
colourless, sweet smelling highly flammable liquid
found in naturally crude oil, component of petrol & found in cigarette smoke
classified as a carcinogen
What type of hydrocarbon is benzene?
An aromatic hydrocarbon or an arene.
What is the basic structure of benzene?
Hexagonal ring of 6 carbon atoms with each carbon bonded to one hydrogen & 2 other carbons.
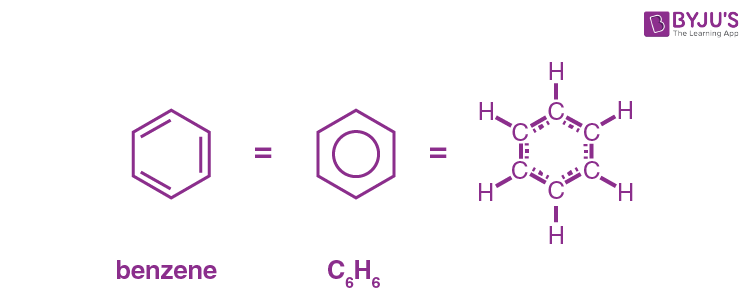
What is the molecular formula of benzene?
C6H6
Why was it a struggle for scientists to make a correct model of benzene from its molecular formula & the experimental evidence initially?
The molecular formula C6H6 suggested a structure containing many double bonds or triple bonds.
Double bond containing compounds were known to be very reactive, however benzene appeared unreactive.
What is the Kekule model of benzene?
Benzene is a six membered ring of carbon atoms joined by alternate single & double bonds.
What are the main pieces of evidence used to disprove Kekule’s model of benzene?
The lack of reactivity of benzene
The length of carbon-carbon bonds in benzene
Hydrogenation enthalpies
Why is the lack of reactivity of benzene evidence to disprove Kekule’s model?
If benzene contained the alternate C=C bonds it should decolourise bromine in an electrophilic addition reaction however:
Benzene doesn’t undergo electrophilic addition reactions
Benzene doesn’t decolourise bromine under normal conditions
This has led scientists to believe benzene doesn’t have any C=C bonds.
Why is the lengths of the carbon-carbon bonds in benzene evidence to disprove Kekule’s model?
The use of X-Ray diffraction allows scientists to measure bond lengths in a molecule. When benzene was examined in 1929 it was found all the bonds in benzene had a length of 0.139nm which is between the length of a single bond and a double bond.
Why are the hydrogenation enthalpies of benzene evidence to disprove Kekule’s model?
If Kekule’s model was correct it could be given a name containing cyclohexene which means we can compare it’s hydrogenation enthalpies to cyclohexene.
It would be expected to have an enthalpy change of hydrogenation that is 3x that of cyclohexene - which has an enthalpy change of -120 - so one of -360.
However the actual enthalpy change is -208 meaning the actual benzene model is more stable than the Kekule one.
What did the contrasting evidence against Kekule’s model for benzene lead scientists to propose?
The delocalised model of benzene.
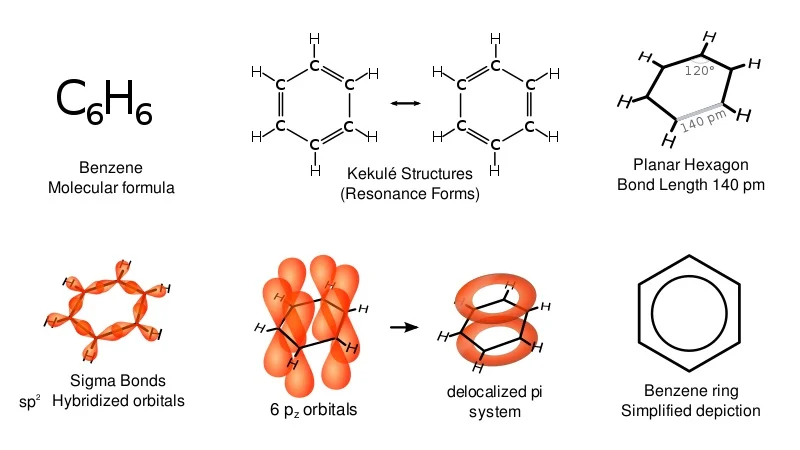
What is the general structure of benzene in the delocalised model?
Benzene is a planar, cyclic, hexagonal hydrocarbon containing six carbons and six hydrogens
How does the delocalised model of benzene work?
Each carbon uses 3 of its available 4 electrons in bonding to 2 other carbons and one hydrogen
Each carbon has one electron in a p-orbital perpendicular to the plane of the bonded carbons & hydrogens
Adjacent p-orbital electrons overlap sideways in both directions - above and below the plane of the carbons to form a ring of electron density
This overlapping creates a system of pi bonds which spread over all 6 of the carbons in the ring structure
The 6 electrons in the pi bonds are said to be delocalised
What are the different substituent groups of benzene that use a prefix?
Short alkyl chains - methyl, ethyl etc
Halogens - fluoro, chloro , bromo, idodo
Nitrogen - nitro
When is a group bound to benzene a substituent?
When the group contains less then 7 carbons or isn’t a carbon chain with an alkyl group e.g. an alcohol group.
The benzene ring is considered to be the parent chain.
When is benzene not considered a parent chain if a group is bound onto it?
When a benzene ring is attached to an alkyl chain with a functional group or to an alkyl chain with 7 or more carbon atoms - in these cases benzene is considered the substituent.
Instead of benzene the prefix phenyl is used in the name.
What are some exceptions to the rule for naming benzene etc that need to be remembered?
Benzoic acid - bezenecarboxylic acid
Phenylamine
Benzaldehyde - benzenecarbaldehyde
How do you name a benzene compound with multiple groups?
Same idea with a carbon chain where the substituent groups are listed in alphabetical order using the smallest numbers possible.
What is the typical reaction of benzene to form a derivative?
Electrophilic substitution where one of the hydrogens is substituted with another group.
What are the different electrophilic substitution reactions that benzene undergoes?
Nitration - to form a nitrobenzene
Halogenation - to form a “halo”benzene
Alkylation - to form an “alkyl”benzene
Acylation - to form something like phenylethanone
What reactants & conditions are needed for a nitration of benzene reaction to occur?
Benzene (duh)
HNO3
Catalyst - H2SO4
Temperature below 50 degrees Celsius
What is the group that needs to bind to benzene in a nitration reaction?
NO2+
How is NO2+ formed in a nitration of benzene from the original reactants & conditions?
It is formed in situ -
H2SO4 + HNO3 —> HSO4- + H2NO3+
This happens because the nitric acid is a weaker acid than the sulfuric acid so acts as a base in this reaction
The H2NO3+ is very unstable to splits apart to form H2O & NO2+ giving the NO2+ electrophile required for the reaction.
H2NO3+ —-> H2O + NO2+
Why is a temperature below 50 degrees celsius important to the nitration of benzene?
If it was above this could lead to multiple nitrations which is especially a problem in the nitration of methylbenzene as multiple nitrations can form Trinitrotoluene aka TNT.
What is the overall reaction of the nitration of bezene?
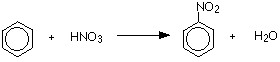
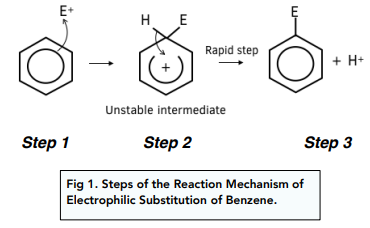
What is the mechanism for electrophilic substitution reactions of benzene?
The electrophile E+ is attracts pair of electrons in the delocalised p-orbital electron structure forming a bond
An intermediate is formed where the delocalised structure is disrupted and both the hydrogen and E are bound onto the benzene
This intermediate ion has a partially delocalised structure but away from the carbons close to/bound to H & E
The ion has a positive charge because the E+ was positive
The hydrogen will then bind to a nucleophile in the reaction mixture - e.g. O: - this doesn’t need to be represented
This means the 2 electrons in the dative hydrogen bond with benzene are no longer in the bond and so they move down to plug the gap in the delocalised electrons restoring the delocalised ring of electrons
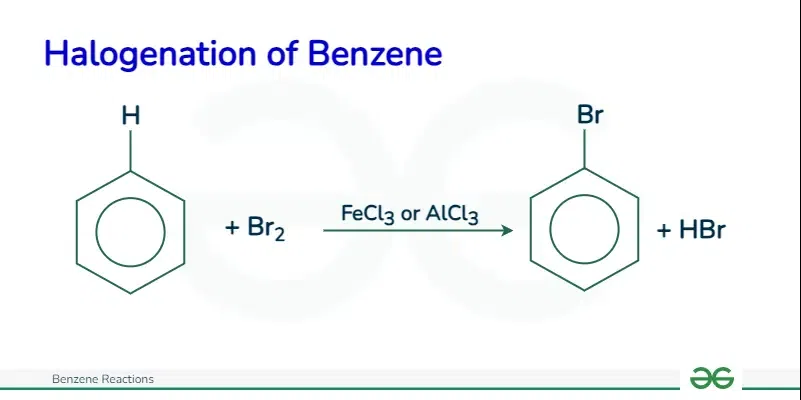
What are the reactants & conditions required for the halogenation of benzene?
Benzene (duh)
The halogen e.g. Cl2
A corresponding iron or aluminium compound catalyst e.g. FeCl3 or AlCl3
This can also be formed in situ from a reaction between the metal and halogen
What is the electrophile in a halogenation reaction of benzene?
Cl+ or Br+ etc
How is the Cl+ or Br+ etc formed in the halogenation of benzene?
AlCl3 + Cl2 —> AlCl4- + Cl+
What is the overall reaction for the halogenation of benzene?
How is the catalyst reformed in the halogenation of benzene?
AlCl4- + Cl2 —> AlCl3 + HCl
The H is from the benzene
What can the catalyst used in halogenation & alkylation of benzene reactions also be called?
A halogen carrier.
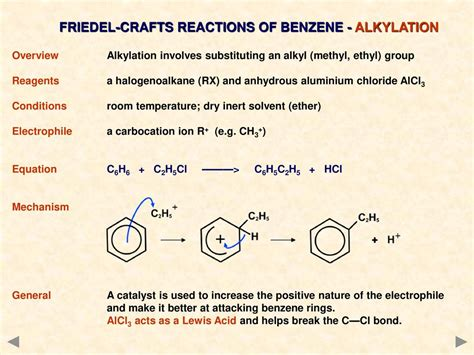
What reactants and conditions are needed for the alkylation of benzene?
Benzene (duh)
An haloalkane - CH3Cl
An catalyst - AlCl3
What is the electrophile required for the alkylation of benzene?
A carbocation - CH3+
How is an electrophile generated from the reactants in an alkylation of benzene reaction?
AlCl3 + CH3Cl —> CH3+ + AlCl4-
What is the overall reaction for the alkylation of benzene?
How is the catalyst in an alkylation of benzene reaction reformed?
H+ + AlCl4- —> HCl + AlCl3
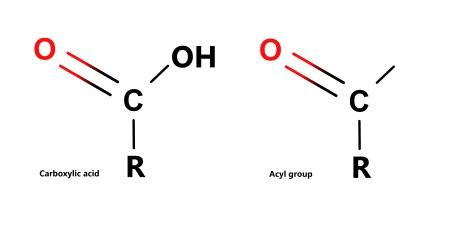
What is an acyl group?
A carbon double bonded to oxygen and bonded to a hydrogen with one bond left unbonded which can have a specific group attached to it e.g. chlorine.
What are the reactants and condition needed for an acylation of benzene reaction?
Benzene (duh)
An acyl chloride - CH3COCl is the first member of the series
A catalyst - AlCl3
What is the electrophile required in the acylation of benzene?
CH3COCl + AlCl3 —→ AlCl4- + CH3CO+
What is the overall reaction for the acylation of benzene?
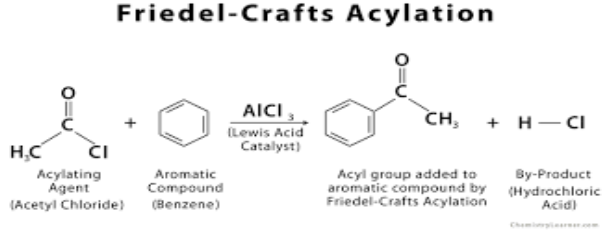
How is the catalyst used in an acylation of benzene reaction reformed?
AlCl4- + H+ —> AlCl3 + HCl
What are the organic products formed from the electrophilic substitution reactions that benzene undergoes?
Nitration of benzene - nitrobenzene
Halogenation of benzene - “halo”benzene
Alkylation of benzene - methyl/ethylbenzene
Acylation of benzene - an aromatic ketone e.g. phenylethanone
How are alkylation & acylation reactions of benzene different to nitration and halogenation reactions?
In alkylation & acylation reactions of benzene a carbon chain is added so there is a new carbon bond is formed increasing the number of carbon atoms in the compound.
What is an alkene vs an arene?
An arene is the equivalent aromatic compound of an alkene e.g. cyclohexene and benzene.
Alkenes undergo electrophilic addition reactions (to break the double bond) whereas arenes undergo electrophilic substitution reactions (there are no double bonds to break and if a C-C bond was broken it would disrupt the delocalised structure)
Why is cyclohexene able to react with bromine without a catalyst while benzene cannot?
Benzene has delocalised pi electrons spread above and below the plane of the carbon ring
Cyclohexene has its electrons localised in single C=C bonds
This means that the electron density between any 2 carbons in benzene is less than the electron density between one C=C bond in cyclohexene
Halogen molecules are non-polar so need to be polarised by electron density to form a delta positive halogen
Cyclohexene can do this without a catalyst due the higher electron density that benzene doesn’t have
This is why benzene needs a catalyst or halogen carrier
What do ortho, para and meta mean in substitution reactions of bezene?
They are positions where another substituent group can bind onto relative to the ‘first’ substituent X.
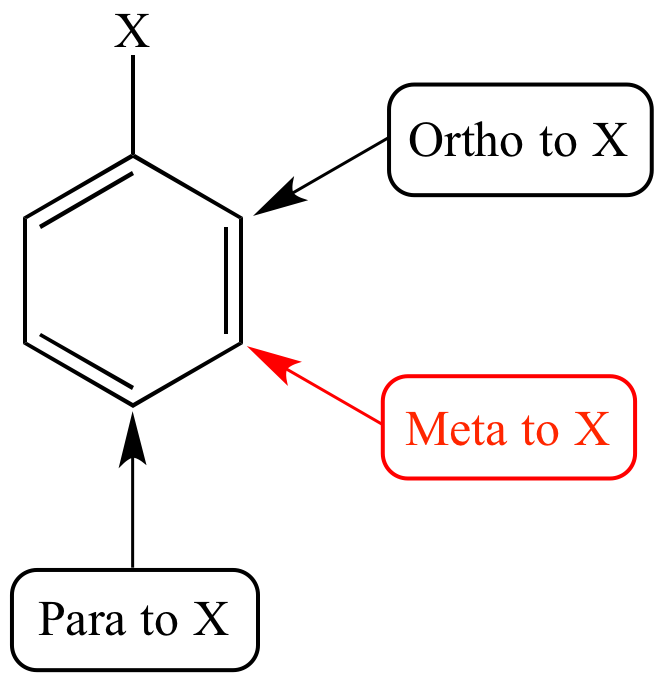
What does it mean for a benzene with a substituent to be activated or deactivated?
A further substituent added onto a benzene (with one already) can activate or deactivate the ring - the rate of reaction is sped up or slowed down.
The activation or deactivation also limits which positions the further substituent can take.
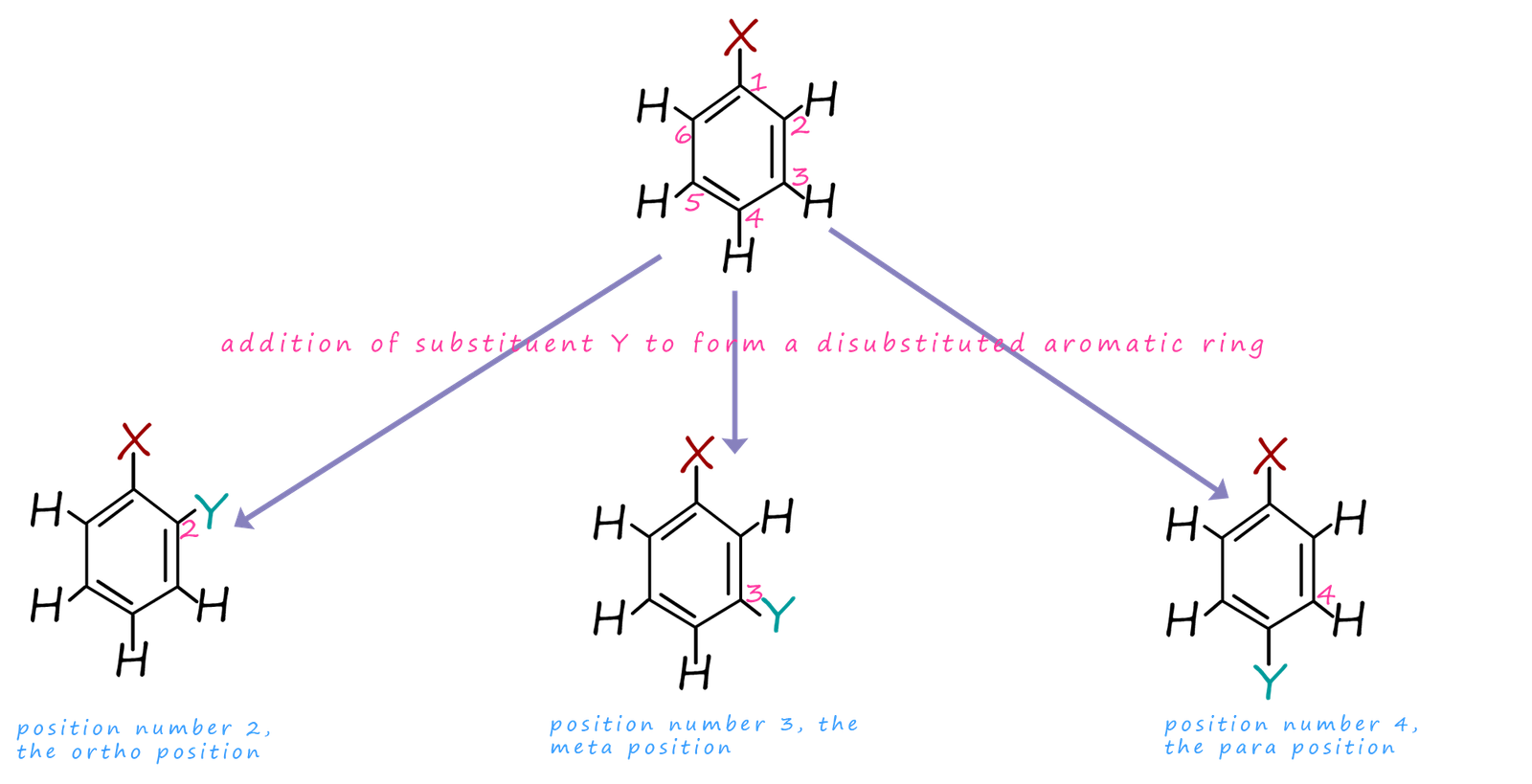
What does an activated benzene ring look like?
The second substituent group can form bonds at ortho and para so at carbons 2,6, & 4.
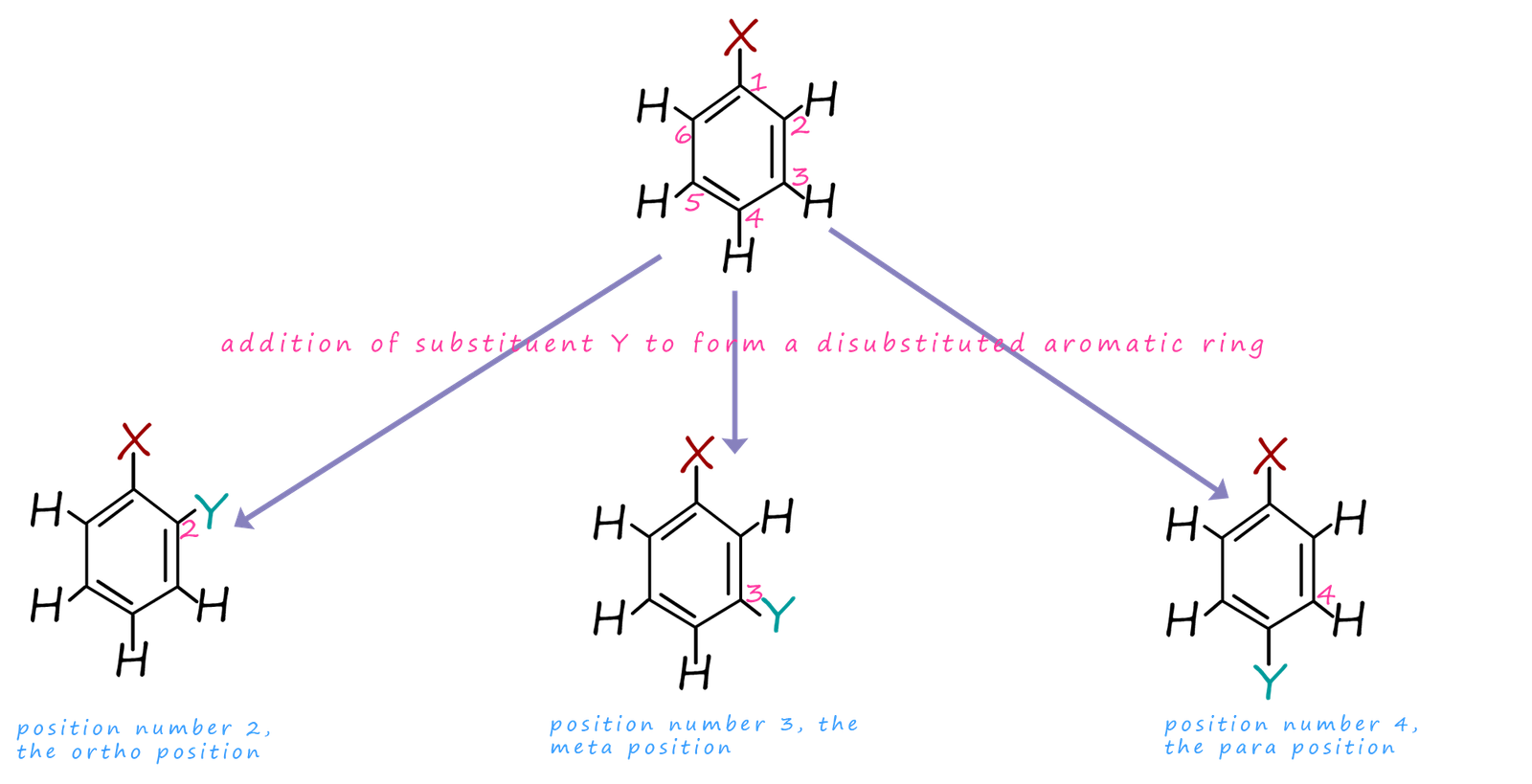
What does a deactivated benzene ring look like?
The second substituent group can form bonds at meta so at carbons 3 & 5.
Why is there a faster rate of reaction when benzene is activated?
There is a higher electron density donated to the delocalised benzene ring.
What are some activating substituents?
NH2, OH, OCH3, CH3
Why do activating substituents activate benzene rings?
They all have lone pairs of electrons in their structure which can overlap with p orbitals to join in/donate electron density to the delocalised structure.
CH3 doesn’t have any lone pairs but still acts as an activating substituent because it has 9 total electrons - still less than the other ones but more so than just hydrogen.
What are some examples of deactivating substituents?
NO2,C≡N, CO2CH3,Cl
Why do deactivating substituents deactivate benzene rings?
They are all have positive charged on the atom that bonds to the benzene ring due to being bonded to a highly electronegative atom within the group or the group as a whole is highly electronegative.
The slight positive/positive charge or high electronegativity means that electrons are pulled towards the substituent group so the electron density of the ring is lowered. This lowers the overall dipole so the benzene is less reactive with electrophiles.
What is a carbonyl group?
A carbon atom double bonded to an oxygen atom - C=O.
What are the two main carbonyl compounds?
Aldehydes - the C=O is found at the end of the carbon chain.
Ketone - the C=O group is found anywhere but the end of the carbon chain.
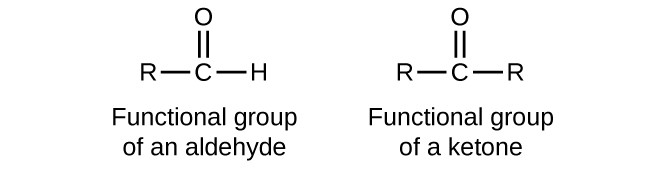
What is the nomenclature for an aldehyde?
The structural formula is written as CHO as to not confuse it for an alcohol - COH.
When the CHO group is on a ring the suffix changed from -al to -carbaldehyde.
If there are other substituent groups they always start numbering from the aldehyde - no need to number it as one.
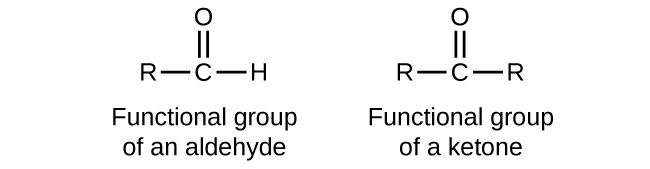
What is the nomenclature for a ketone?
The structural formula is CO and the name always ends in the -one.
Ketones do require a number in their name to identify which carbon the -CO is on unless its a cyclic compound where you start numbering the ring with the carbon bonded to -CO meaning one is often omitted from the name.
What is Brady’s Reagent and why is it useful?
Brady’s Reagent is a solution of 2,4DNP/H in a mixture of methanol and sulfuric acid.
It can be used to detect the presence of carbonyl groups - whether something is an aldehyde or a ketone as in the presence of either a yellow/orange precipitate is formed.
It has a pale orange colour.

How does Brady’s Reagent work?
There is an amine group on 2,4 DNP that reacts with the carbonyl group to form a N=C bond and a derivative of DNP. This forms a precipitate which can be seen.
Why is Brady’s Reagent hazardous?
It’s very volatile as a liquid and as a solid/dry Brady’s Reagent is also very sensitive to friction.
What is the test for carbonyls using Brady’s Reagent?
Add 5cm3 of Brady’s Reagent to a clean test tube
Add a few drops of aldehyde/ketone and leave to stand
A yellow/orange precipitate indicates the presence of an aldehyde or ketone
How can the precipitate from a test for carbonyls using Brady’s Reagent be analysed to determine which carbonyl it is?
Recrystallisation:
The precipitate is filtered then dissolved in the minimum volume of warm solvent
Cooled to cause precipitation then filtered again
You can then compare the melting point to determine the identity of the 2,4 DNP derivative
How can a sample of a carbonyl be identified as a ketone or aldehyde without using Brady’s Reagent?
Reaction with Tollens Reagent - solution of silver nitrate in aqueous ammonia
In the presence of an aldehyde a ‘silver mirror’ will form
How does the formation of a ‘silver mirror’ in a reaction of Tollens Reagent & aldehyde work?
The Ag+ ions are reduced to Ag as the aldehyde is oxidised to form a carboxylic acid - the Ag forms the mirror.
What reactions do carbonyls undergo and why?
They undergo nucleophilic addition reactions.
The polarity of the C=O bond means that the carbon is delta positive and the oxygen is delta negative (due to its higher electronegativity). This attracts nucleophiles towards the carbon atom resulting in addition across the C=O bond.
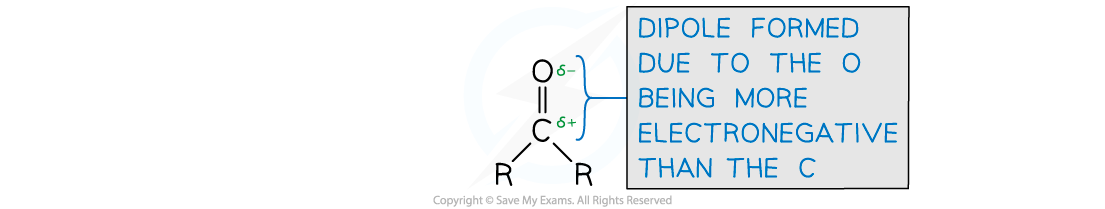
What is the arrangement of atoms and electrons in a carbonyl group?
The arrangement of atoms is trigonal planar around the carbon.
The electrons in the bond are the same as a C=C bond - sigma bond on the plane of C & O and the pi bond above and below.
What are the nucleophilic addition reactions undergone by both ketones and aldehydes?
Reaction with a reducing agent - most often sodium tetrahydridoborate, NaBH4.
This forms an alcohol - 2nd alcohol with a ketone & primary alcohol with an aldehyde.
Nucleophilic addition with HCN
Forms a longer carbon chain with both an alcohol/hydroxy group and a -CN nitrile group making it useful in organic synthesis
What does the reaction between carbonyls and NaBH4 look like & what are the reaction conditions?
The carbonyl is usually warmed with the NaBH4 in aqueous solution.


What happen in a nucleophilic addition mechanism in the carbonyl reaction with NaBH4 ?
Nucleophilic attack on the carbonyl group to form negatively charged intermediate by the nucleophile - :H- /hydride ion contained in NaBH4
The lone pair of electrons on :H- is attracted & donated to the delta positive carbon in C=O
A dative covalent bond is formed between the hydride & carbon
The pi bond in C=O breaks by heterolytic fission forming a negatively charged intermediate
This intermediate has a :O- that donates a pair of electrons to a delta positive hydrogen in a water molecule
the reaction is aqueous which is where the water is from
The intermediate has been protonated to form an alcohol
What does the nucleophilic addition mechanism in the carbonyl reaction with NaBH4 look like?


What are the reactions conditions of a carbonyl & HCN nucleophilic addition reaction?
Sodium potassium cyanide & Sulfuric acid are used to react with the carbonyl instead of HCN as it is a colourless, extrememly toxic liquid that boild at 20C and can’t be used in an open lab.
Sodium potassium cyanide & Sulfuric acid will form HCN and while still hazardous the reaction isn’t as bad.
What is the mechanism of the nucleophilic addition reaction between a carbonyl & HCN and how is this different to a reaction with NaBH4?

The H+ that protonates the negative intermediate can come from water from the aqueous solution or HCN.
What does the reaction between carbonyls and HCN look like?
What are carboxylic acids?
Organic acid which contains the carboxyl functional group RCOOH. The carboxyl functional group is made up of both a carbonyl group (C=O) and a hydroxyl group (-OH)
How do carboxyl groups differ from carbonyl groups?
The carboxyl group also contains a hydroxyl/-OH group where as carbonyl groups only have C=O and this effects the way they react.
Because both the C=O & O-H bonds are polar carboxyl groups experience dipole-dipole interactions with themselves & water etc - hydrogen bonds.
What are the properties of carboxylic acids?
Soluble in water & polar solvents - the hydrogen bonding can form with polar solvent molecules e.g. water but this solubility decreases as the chain length increases since the hydrophobic part of the molecule increases
Ability to form dimers - in a pure carboxylic acid sample, hydrogen bonding can occur between 2 carboxylic acids forming a dimer
Increased boiling & melting point - due to hydrogen bonding
Act as weak acids - carboxylic acids can partially dissociate the H from the OH bond, but this reaction can be undone/is reversible
RCOOH ⇌ RCOO- + H+
What non-organic reactions can carboxylic acids undergo?
Redox reactions with metals
Neutralisation reactions with bases to form carboxylic salts
What happens in a redox reaction of a carboxylic acid and metal?
RCOOH(aq) + Na(s) —> RCOO-Na+(aq) + H2(g)
Carboxylic acid + Metal —> Carboxylate salt + Hydrogen gas
What happens in a neutralisation reaction with a carboxylic acid?
RCOOH(aq) + NaOH(aq) —> RCOO-Na+(aq) + H2O(l)
Carboxylic acid + base/alkali —> Carboxylate salt + Water
How can you test for a carboxyl group?
Neutralisation with a metal carbonate to form a salt, water and carbon dioxide.
This is because carboxylic acids are the main/only organic compounds acidic enough to neutralise bases - phenol is slightly acidic but not acidic enough to react with carbonate
Carbon dioxide gas is formed and if the acid is in excess a solid metal carbonate would disappear
What are carbxoylic acid derivatives?
Compounds that can be hydrolysed to form a parent carboxylic acid.
All carboxylic acid derivatives contain a sequence of atoms called the acyl group.
What is an acyl group?

Acyl group have the same structure as a carboxyl group except the -OH group is replaced with an R etc.
What are some example of carboxylic acid derivatives?
Esters
Acyl Chlorides
Acid Anhydrides

What is an ester?
An ester is a carboxylic acid derivative where the H in COOH is replaced with an alkyl chain - this could be an alkyl group e.g. methyl, ethyl or an aromatic group e.g. phenyl.

How does esterification occur?
Ester formation/esterification is the reaction of an alcohol with a carboxylic acid to form an ester.
Esterification reactions are condensation ones that eliminate water and are reversible, quite slow and have low yields.
What does an esterification reaction look like?
Ethanol + Propanoic acid —> Ethyl Propanoate + Water

What are the reaction conditions for esterification reactions?
Conc Sulfuric acid as a catalyst & heating under reflux.
How do you name esters?
An ester is named after the parent carboxylic acid from which it is derived
Remove the -oic acid suffix from the parent carboxylic acid and replace with -oate
The alkyl chain attached to the oxygen atom of the -COO- group is then added as the first word in the name
This part of the name comes from the alcohol, e.g. propanol becomes propyl
Ester names are confusing because the name is written backwards from the way the structure is drawn
Methyl propanoate = methyl + propanoic acid

What are phenols?
C₆H₆ rings bonded directly with OH⁻ groups.
How are phenols different to other (aliphatic) alcohols?
Phenol is less soluble in water than alcohols due to the presence of the non-polar benzene ring. Phenol is also more acidic than other alochols.
How does phenol act as a weak acid?
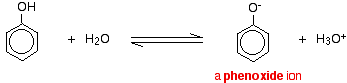
When dissolved in water, phenol partially dissociates forming the phenoxide ion and a proton. Because of this ability to partially dissociate to produce protons, phenol is classified as a weak acid.
How is phenol stable enough as phenoxide to act as a weak acid?
The negative charge on the oxygen atom is delocalised around the ring. One of the lone pairs on the oxygen atom overlaps with the delocalised electrons on the benzene ring leading to a delocalisation which extends from the ring out over the oxygen atom. As a result, the negative charge is no longer entirely localised on the oxygen but spread out around the whole.
Spreading the charge around makes the ion more stable than it would be if all the charge remained on the oxygen.
However oxygen is the most electronegative element in the ion and the delocalised electrons will be drawn towards it. That means that there will still be a lot of charge around the oxygen which will tend to attract the hydrogen ion back again.
That's why phenol is only a very weak acid.
How can we differentiate carboxylic acids & phenols if we know our sample is an acidic organic compound?
Phenols and carboxylic acids react with solutions of strong bases such as aqueous sodium hydroxide. Only carboxylic acids are strong enough acids to react with the weak base, sodium carbonate.
A reaction with sodium carbonate can be used to distinguish between a phenol and a carboxylic acid. The carboxylic acid reacts with sodium carbonate to produce carbon dioxide, which is evolved as a gas.
What types of bases can phenol react with?
Only strong bases as phenols are weak acids so aren’t strong enough to react with weak bases e.g. sodium hydroxide vs sodium carbonate & hydrogencarbonate solutions.
What types of reaction can phenols undergo?
Neutralisation reactions due to its property of being a weak acid
Electrophilic subistution reactions with halogens & nitrate
What happens in a neutralisation reaction of phenol and sodium hydroxide?
Phenol reacts with sodium hydroxide solution to give a colourless solution containing sodium phenoxide.
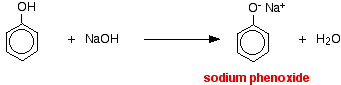
In this reaction, the hydrogen ion has been removed by the strongly basic hydroxide ion in the sodium hydroxide solution.
What happens in a bromination reaction of phenol?
Phenol reacts with Br₂ (aq) to form a white precipitate of 2,4,6-tribromophenol, decolourising it
A halogen carrier catalyst is not required and the reaction is carried out at room temperature.
Electrophilic subsitution
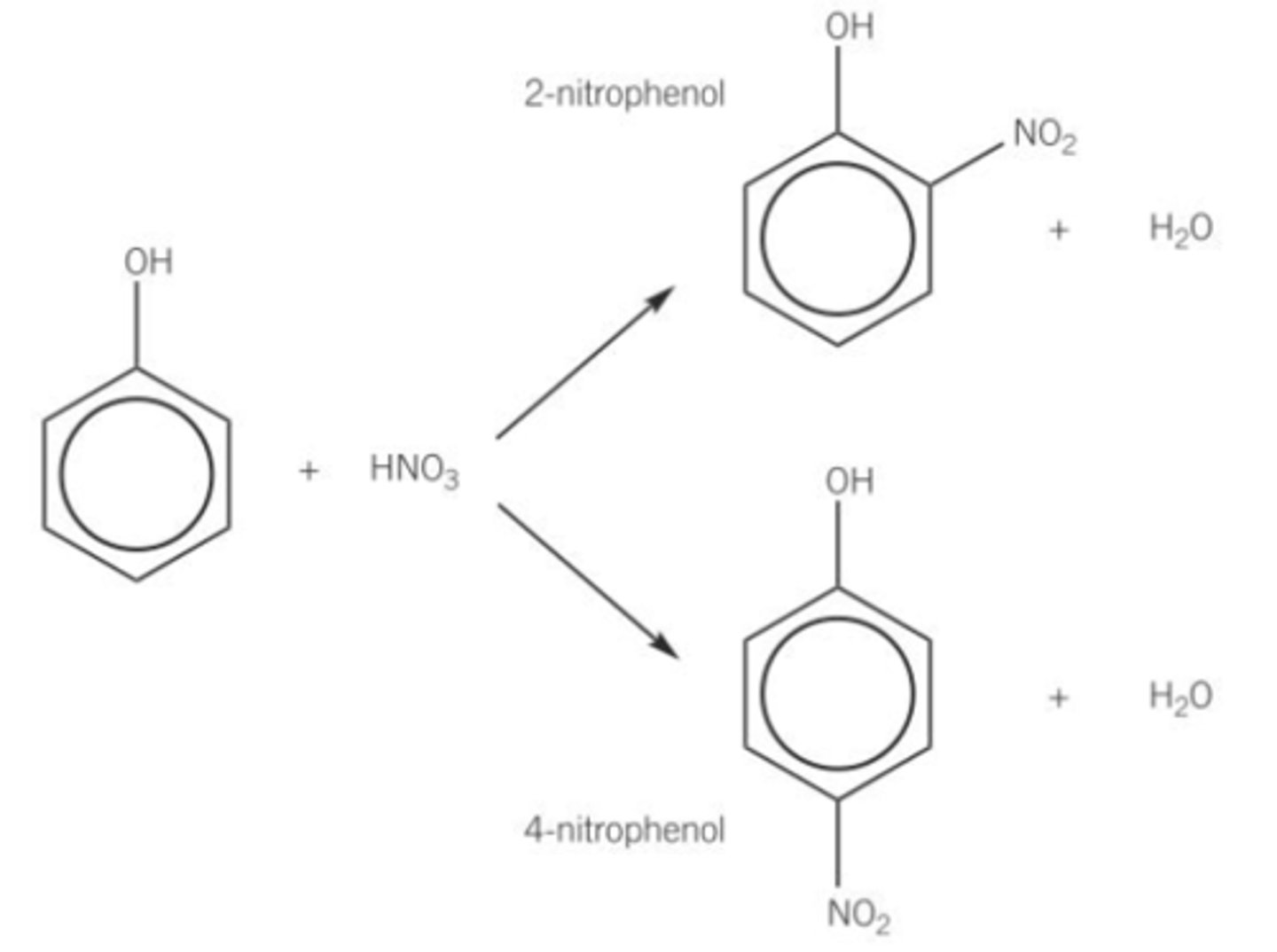
What happens in a nitration reaction of phenol?
Phenol reacts readily with dilute HNO₃ to form a mixture of 2-nitrophenol and 4-nitrophenol.
Reaction occurs at room temperature.
Requires only dilute nitric acid
Electrophilic Substitution reaction.