APES All Formulas and Equations from all units
1/33
Earn XP
Description and Tags
ALL OF THE APES FORMULAS AND EQUATIONS. (atleast the ones needed to pass the exam)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Respiration
C6H12O6 + 6O2 → 6CO2 + 6H2O
chemical reaction in which glucose and oxygen are turned into water, carbon dioxide, and energy
Photosynthesis
6CO2 + 6H2O + sunlight → C6H12O6 + 6O2
six carbon dioxide molecules and six water molecules, are converted by light energy captured by chlorophyll (implied by the arrow) into a sugar molecule and six oxygen molecules, the products.
Combustion
Hydrocarbons (CxHy) + O2 → CO2 + H2O
the reaction of a hydrocarbon (only C and H) with oxygen gas (O2) The reactants are CxHy and O2. The products are CO2 and H2O.
Ocean Acidification
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3-
CO2 reacts with water to form carbonic acid, Carbonic acid can then dissociate into bicarbonate (HCO3), Bicarbonate can then dissociate into carbonate ions (CO3),
Photochemical Smog Formation
NOx + VOCs + heat + sunlight → smog
NOx from Fossil fuel combustion gets broken down by sunlight into NO+O-. The O- reacts and bonds with O3(Ozone) and makes tropospheric O3, the NO reacts with VOCs and creates photochemical oxidants. Then the O3 and photochemical oxidants bond to make photochemical smog.
Stratospheric Ozone Depletion
CFC’s+UV = Cl-
Cl- + O3 = ClO + O2
ClO + O- = Cl- +O2
CFCS are broken down by UV rays into Cl-. The free Chlorine bonds Ozone and makes ClO + O2. The cycle then repeats and chlorine doesn’t allow the free O2 molecule to bond another oxygen atom.
Stratospheric Ozone Formation
O- + O2 =O3
O3+UV=O- +O2
A free O(oxygen) atom bonds with an O2 (oxygen molecule) and makes stratospheric ozone. The O3 is then broken apart by UV rays into O2 and O- and the free O- bonds to another O2 and creates more O3. The cycle Repeats.
Tropospheric Ozone Formation
ultraviolet sunlight breaks apart an oxygen molecule to form two separate oxygen atoms. In the second step, each atom then undergoes a binding collision with another oxygen molecule to form an ozone molecule.
Acid Rain Formation (NOx)
NOx + H2O = HNO3 (Nitric Acid)
NOx from fossil fuel combustion is released into the atm. and forms chemical reactions with water to create Nitric Acid (HNO3)
Acid Rain Formation (SOx)
SOx + H2O = H2SO4(Sulfuric Acid)
SOx from coal combustion enter the atm. and chemically reacts with water to form Sulfuric Acid(H2SO4).
Population Density
pop./area
Rule of 70 (Doubling time)
70/percent(%) growth rate
population growth rate %
Births - Deaths/Number of people x 100
global population growth rate %
CBR-CDR/10
rate of change
ending-starting/ending time-starting time
percent change
ending-starting/starting x100
Net Primary Productivity
GPP-R=NPP
the rate at which energy is stored as biomass by plants or other primary producers and made available to the consumers in the ecosystem.
Gross Primary Production
GPP=R+NPP
GPP = Total solar energy captured by plants − Energy lost due to respiration by plants
the total chemical energy produced from sunlight through the photosynthesis of plants
Fuel Efficiency
Distance traveled/unit of fuel consumed
10% Rule
Rule states roughly 10% of energy transfers between trophic levels in a food chain, the rest is lost to heat
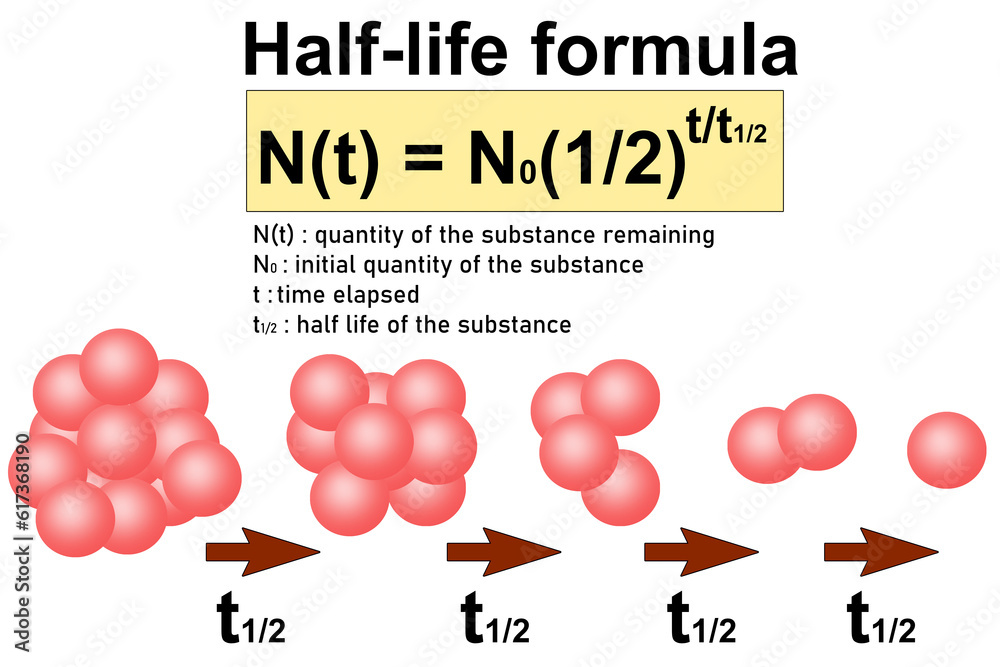
Half-Life
the time required for a quantity (of substance) to reduce to half of its initial value
LD50
dose/weight
Lethal dose (LD50) is the amount of an ingested substance that kills 50 percent of a test sample
Population Growth Rate
(births -deaths+immigrations-emigration)/population x 100
or (crude briths-crude deaths)/10
(emigrations=LEAVING, immigration is COMING/ENTERING POP)
Population Change
(Births-deaths+immigration-emigration)/population
(emigrations=LEAVING, immigration is COMING/ENTERING POP)
Crude Death Rate
(total deaths/ total pop) x 1000
Crude Birth Rate
(total births/total pop) x 1000
Death rate
death rate(%)/100
Death rate (%)
(total deaths/total populations) x 100
Birth rate
birth rate (%)/100
Birth rate (%)
(total births/total population) x 100
Nitrogen Fixation
N2 to NH3
bacteria changes free nitrogen gas into ammonium (nitrogen form)
nitrification
NH3 to NO3
Produces nitrates from ammonia
Ammonia
NH3
Ammonium
NH4