C.Bio - Endocytosis
1/35
Earn XP
Description and Tags
Intro to Endo
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
The lumen space is topologically equivalent to ______
The extracellular matrix
Biosynthetic pathways into the cell?
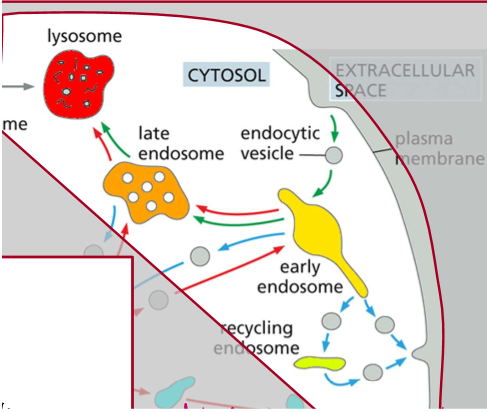
Extracellular space → endocytic vesicle → early endosome → late endosome → lysosome
Biosynthetic pathways out of the cell?
Nuclear envelope → endoplasmic reticulum → golgi apparatus → secretory vesicles → plasma membrane
Roles of endocytosis
Obtain nutrients
Recycle obsolete components
Remove extra membrane
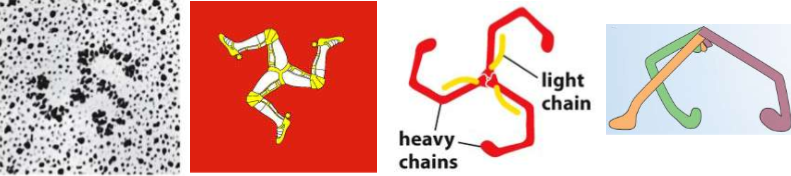
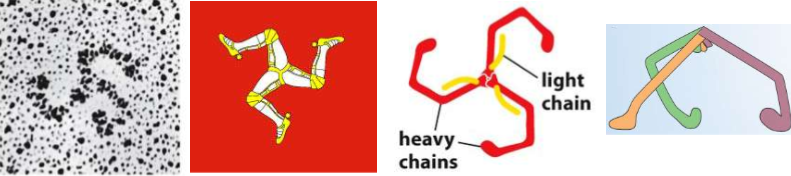
name this shape
TRISKELION
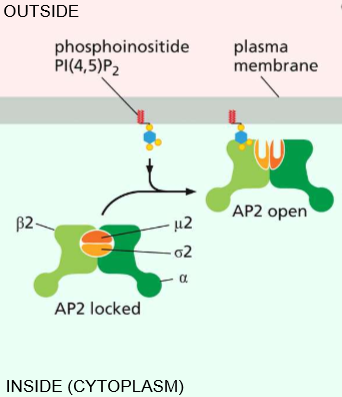
Adapter Protein AP-2 Functions
Recognizing signals as a ligand - They serve as a sorting signal & recruits transmembrane proteins (interacts with PI(4,5)P2!)
Recruit cargo - to prevent pits from forming without contents
Recruit coat - Ex) clathrin recruiting to entice polymerization upon recruitment and bend the membrane
Recruits accessory proteins - Membrane bending proteins by Bar-domain dimer.
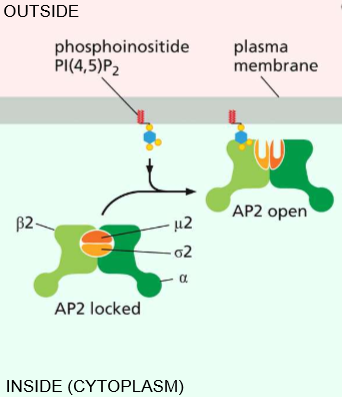
Phosphoinositide (PI(4,5)P2) interaction with AP-2
Essentially activates AP-2 to open & activates its affinity for clathrin.
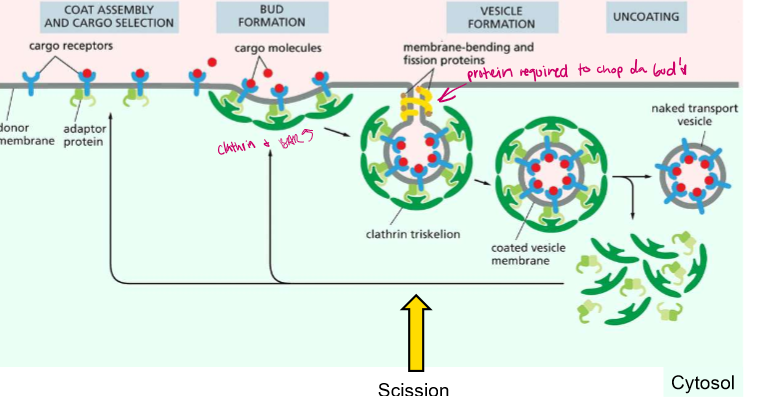
Budding and Scission Steps?
1) COAT AND CARGO ASSEMBLY: AD-2 proteins bind to cargo receptors, which are binding to extracellular cargo
2) BUDDING: Activated AD-2 proteins recruit membrane deformers (clathrin and BAR proteins).
3) BUDDING PT2: Clathrins polymerize and form a coat
4) SCISSION: GTPase Dynamin constricts and orchestrates the fission of the cell membrane from the vesicle
5) The vesicle is freed but still in a clathrin coat
6) Chaperone protein Hsc70 and its cofactor Auxilin liberate the tightly wound clathrin wia ATP hydrolysis
7) The vesicle is freed in the cytosol.
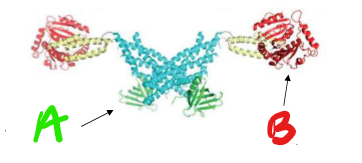
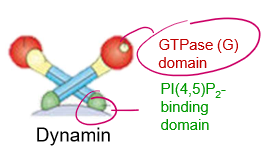
Who’s this pokemon? name the regions
It’s Dynamin!
A) PI(4,5)P2 binding domain
B) GTPase Domain
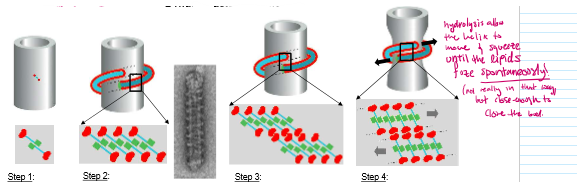
Dynamin-mediated vesicle scission steps
Dynamin binds to PI(4,5)P2
Membrane binding stimulates polymerization into large helixes
G-domains meet and stimulate each other’s GTPase activity→ hydrolyzing GTP
Hydrolysis changes dynamin conformation to make the rungs squeeze closer together
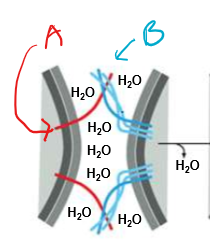
Who’s that pokemon? state their functions and location
A) v-SNARE (vesicle SNARE)
B) t-SNARE (target SNARE)
They anchor the vesicle to the target membrane in order to squeeze out the water shell surrounding both membranes and preventing fusion. Only matching v/t-SNARE pairs are capable of fusion.
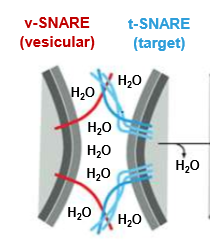
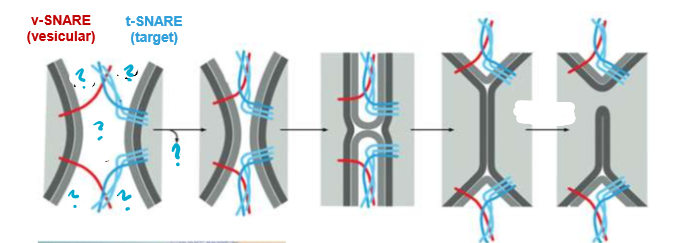
Describe this process
The SNARE proteins are used to bring cells close enough to fuse. The SNAREs will push out water attracted by the hydrophilic shells and thus allow the now adjacent membranes will be allowed to fuse.
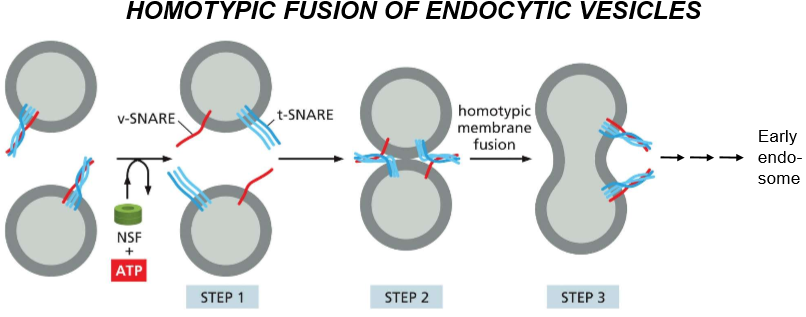
Describe the steps of homotypic fusion of endocytic vesicles and then their recycling process.
Pre-Activity: SNAREs are disentangled and activated by NSF and ATP
SNAREs unentangle and recognize each other
DOCKING: they tangle and push H20 out, causing homotypic membrane fusion
The tangled SNAREs join the membrane of the endosome they’re docked to, forming the trans-SNARE complex
That fusion becomes an early endosome as acids are delivered, and eventually an ATP mediated process via NSF untangles trans-SNARE and chaperones v-SNARE back to it’s original membrane
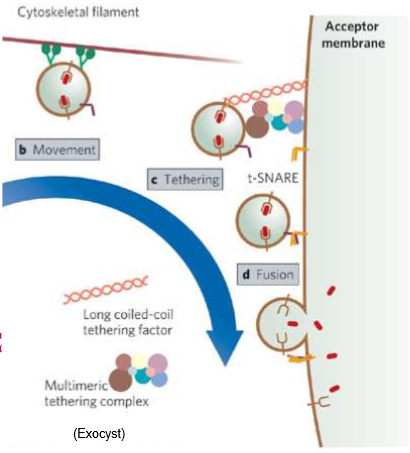
What are tethering proteins?
Cytoskeleton filaments that capture vesicles to help with diffusion.
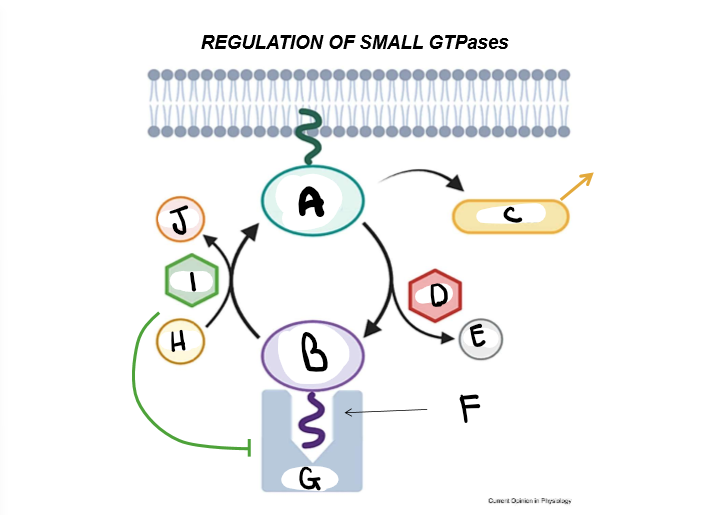
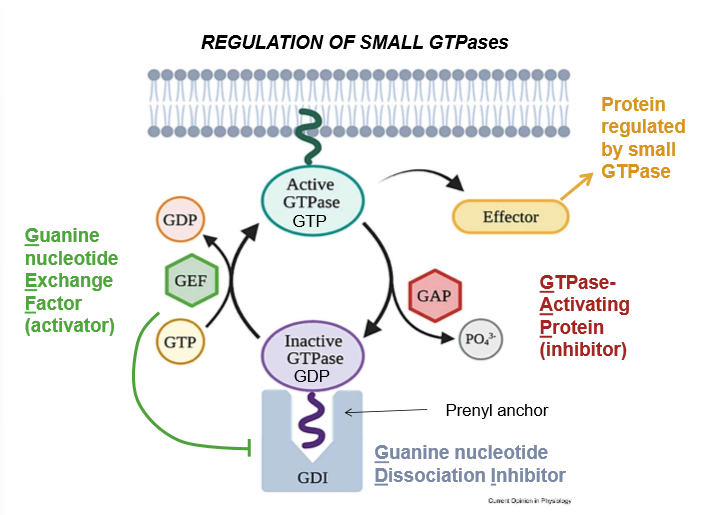
LABELLLL + Purpose
A) Active GTPase - A GTPase with an exposed prenyl anchor, and thus the ability to activate effectors
B) Inactive GTPase - A GTPase with a hidden prenyl anchor and thus, no effector-activating abilities
C) Effector Proteins - proteins that are activated by GTPase in order to perform a myriad of cell functions
D) GAP (GTPase Activating Protein) - Inhibitor of GTPase: It hydrolyzes the GTP that activates GTPase, and upon it’s removal, shuts the larger enzyme down. Works as a regulator
E) Inorganic Phosphate - The cleaved portion of many cell energy carriers.
F) Prenyl anchor - A lipid tail that conformationally allows the activation of GTPase and it’s association with the cell membrane
G) GDI (Guanine Nucleotide Dissociation Inhibitor) - A complex that hides the prenyl tail
H) GTP (Guanine Triphosphate) - Like ATP, this is a nucleobase-energy carrier used to activate many cell signaling pathways.
I) GEF (Guanine nucleotide Exchange Factor) - An activator of GTPase. It kicks out GDI to expose the prenyl factor and allow a place for GTP to bind and activate the GTPase
J) GDP - Guanine diphosphate: Hydrolyzed GTP
List the 6 pieces of Budding Machinery in Endocytosis and examples we’ve used in class
Signalling Molecules (PI(4,5)P2)
Adaptor Proteins (AP-2)
Coat Proteins (Clathrin)
Membrane deforming proteins (BAR domains)
Scission Proteins (Dynamin)
Recycling/Chaperone proteins (HSC70 & auxilin)
List the 4 pieces of Fusion Machinery
t-SNAREs and v-SNAREs
Rab GTPases
Rab Effectors (like tethering molecules)
Recycling/Chaperone Molecules (NSF)
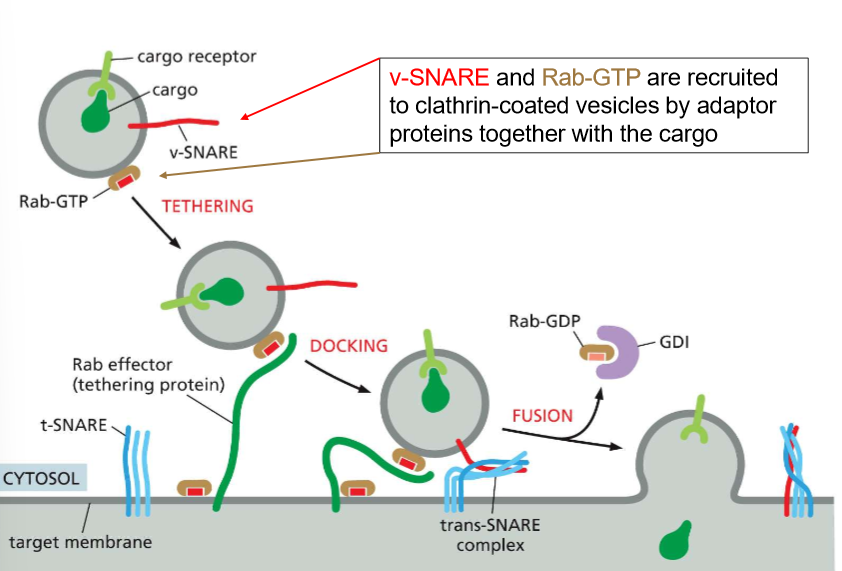
How do Rab-GTP and Rab effector proteins help with the specificity of vesicle tethering?
These are essentially recognizing molecules
Rab-GTP binds to the vesicle and then allows for specific Rab effectors on the target membrane to recognize the vesicle and DOCK.
Rab effectors facilitate the meet-cute of the v-SNARE and t-SNAREs on the surface of the target membrane
When the vesicles begin to fuse, Rab-GDP binds to GDI and returns to it’s donor molecule.
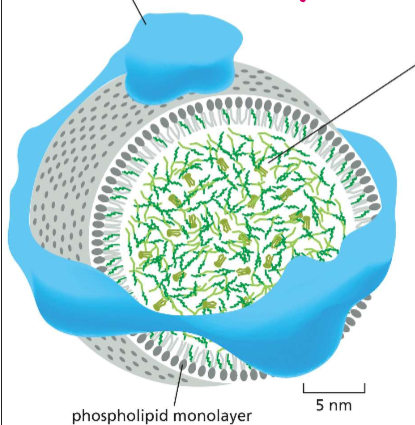
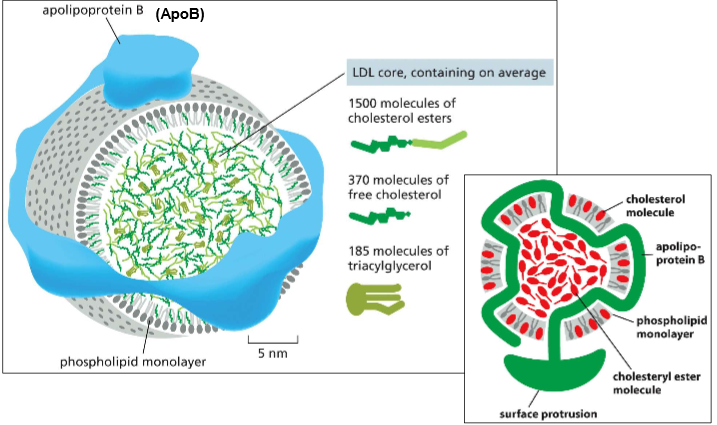
BRO! WHO IS LDL?!?
Low-Density Lipoprotein Particles:
The packaged vesicle made for transporting cholesterol into the cell.
Features:
Phospholipid monolayer (to allow the interior to be hydrophobic)
ApoB (apolipoprotein B) to hold it together
What motif on the LDL receptor does AP-2 recognize
The NPxY motif for Arginine, Proline, Some random Amino Acid, and Tyrosine.
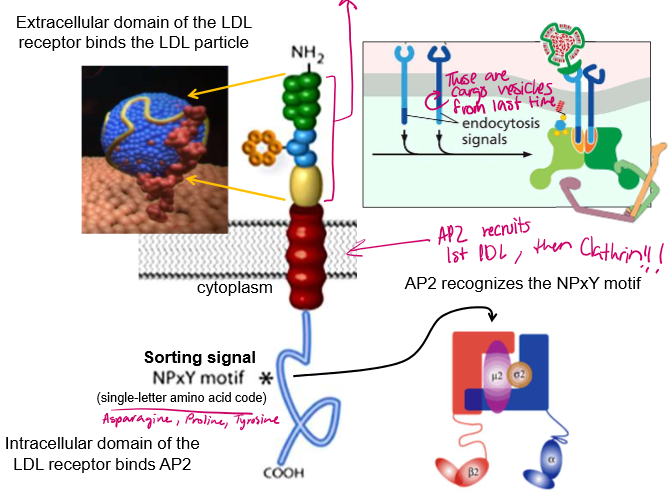
What kind of mutations can disturb the endocytosis of LDL? What are their effects?
ApoB proteins disintegrate and release cholesterol if not taken into the cell in a timely manner:
Defective NPxY mutation in the receptors → AD-2 cannot recruit the proteins
Defective ApoB→ the ApoB shell cannot bind to the LDL-binding site
Defective ApoB binding surface in LDL receptor → receptor cannot recruit LDL particles because of a mishapen binding site
All of these will ultimately lead to high cholesterol
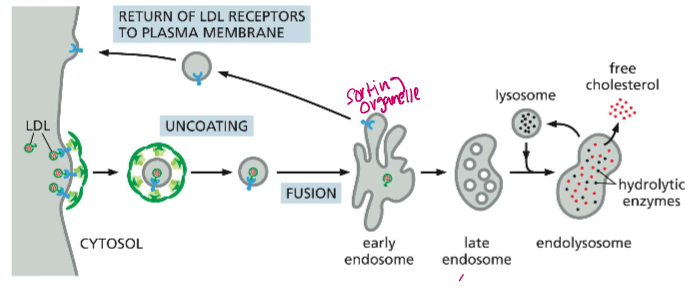
Give an overview of the 7 distinct steps of LDL Endocytosis and Recycling
LDL uncoating - The clathrin coat comes off (Hsc70 & auxilin) once the LDL vesicle is inside the cytosol
Fusion - Fusion into the early endosome
Acidification - H+ pump (delivered from the Golgi) reduces the pH of the endosome to ~6.5
Dissociation: LDL receptor loses affinity for the particle at high pH
Sorting: Empty LDL receptors are sorted into clathrin-coated buds in another section of the endosome and pinched off
Fusion II: Uncoated recycling vesicles fuse to dorm a recycling endosome
Recycled: Empty LDL receptors are plopped back on the membrane.
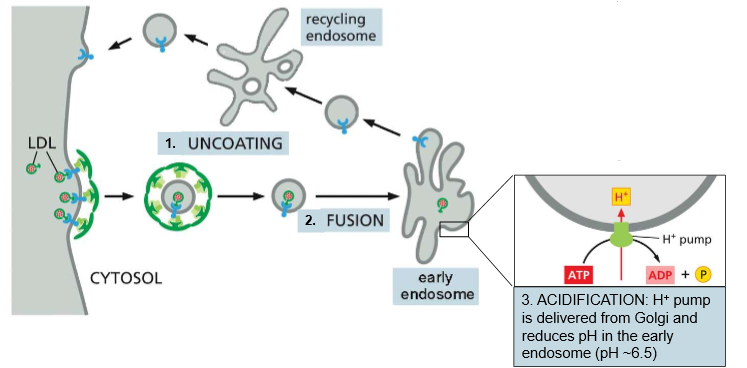
Essentially, all endocytosis vesicle→ endosome movement is driven by _______
pH differences
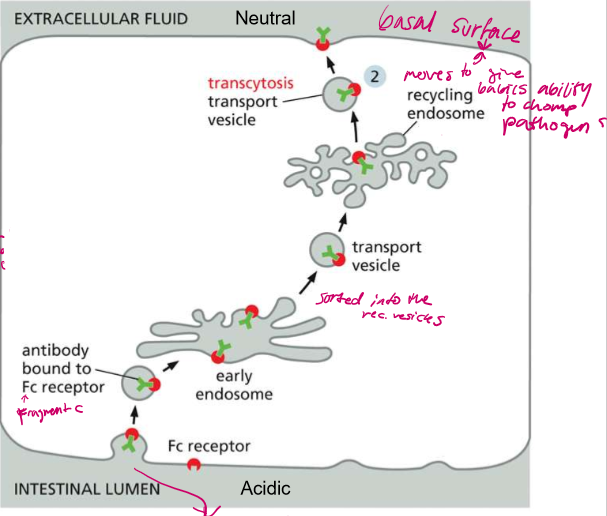
To whom does this process belong?
Epithelial cells in the lumen
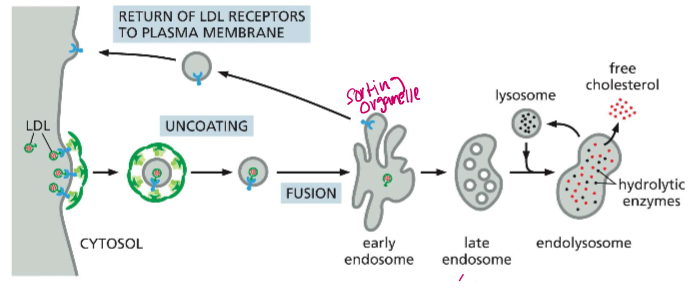
Determine the lifecycle of an endosome in LDL endocytosis
Early endosome: first intact of the LDL particle, dissociates it from the LDL receptor and returns it to the membrane, pH ~6.5
Late endosome: Now filled with LDL particles and acid hydrolases (nucleases, proteases, lipases etc) from the golgi, ph ~4.5
Endolysosome: Now the acid hydrolases are activated. They degrade ApoB and release free cholesterol to other membranes
Lysosome: After the cholesterol is distributed, all that is left is an acidic lysosome chock full of acid hydrolases.
All of these processes are governed by H+ pumps into each phase, causing the maturation of the endosome.
What are the two pathways for receptor-mediated endocytosis?
Recycling (for nutrients and their receptors) or degradation (for signalling receptors)
Why can’t you just stop a RTK (receptor tyrosine kinase) without endocytosis?
The girlies have a high affinity for the growth factors they’ve bound to.
Do RTKs still signal while endocytosed and in the cell?
YES. They continue to be bound to the growth factors and stay phosphorylated until they are marked for degradation by Ubiquitin, which recruits the protein ESCRT to invaginate the cell membrane with Ubiquitin inside.
Which PIP marks endosomes?
PI(3)P, which is on the outer surface of the early endosome
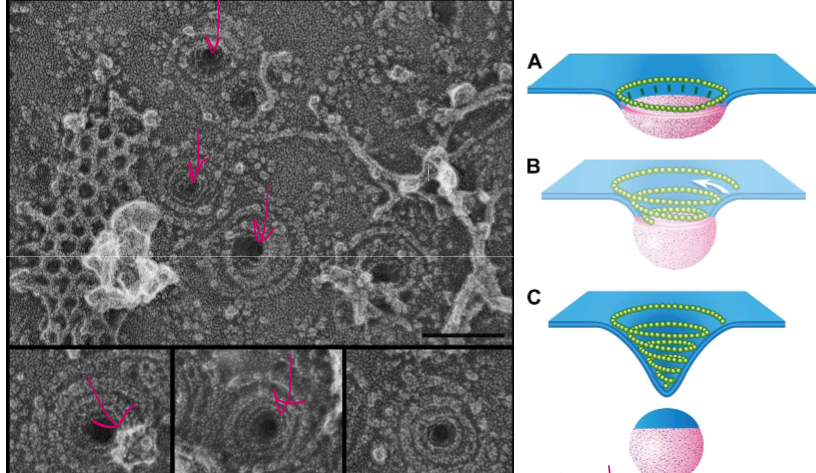
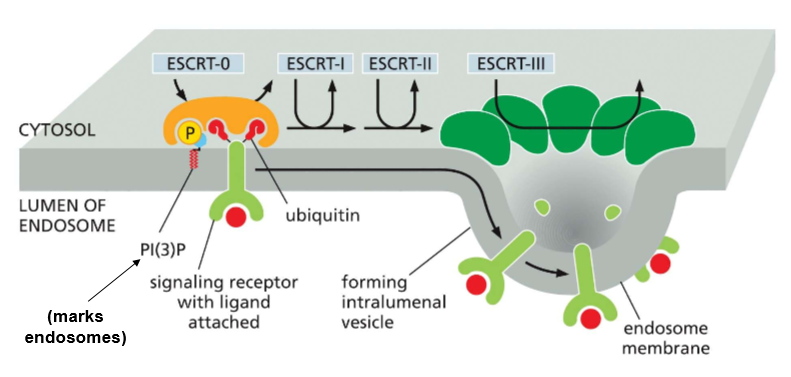
What do ESCRT proteins do?
When ubiquitin and a receptor are present on the surface of an endosome, they are attached by PI(3)P to bind with ESCRT-0, who recruits ESCRT-1, who recruits ESCRT-2, who recruits ESCRT-3 for oligermization into a helix that pulls the membrane in on itself
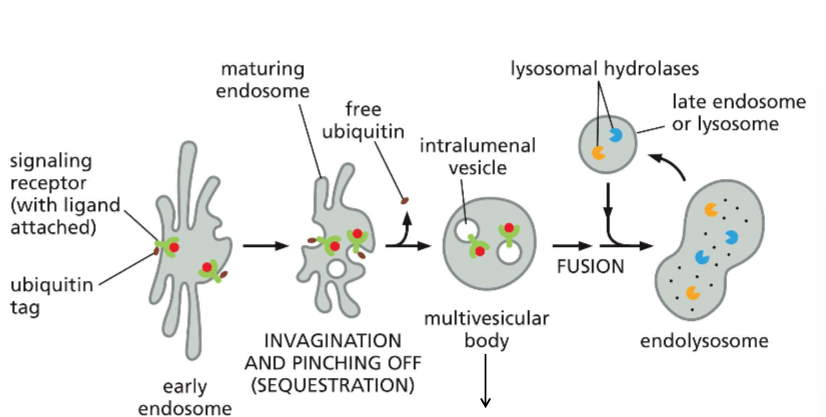
Describe the pathway of endocytosis of RTKs dealing with these growth factors.
Early endosome: Signal attached to ubiquitin here
Maturing endosome: Allows for contact. Will invaginate and pinch off.
Multivesicular Body: same as late endosomes.
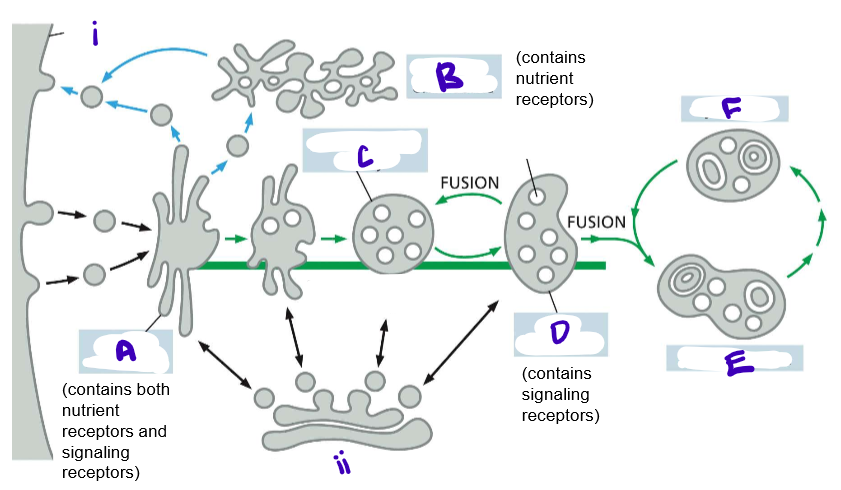
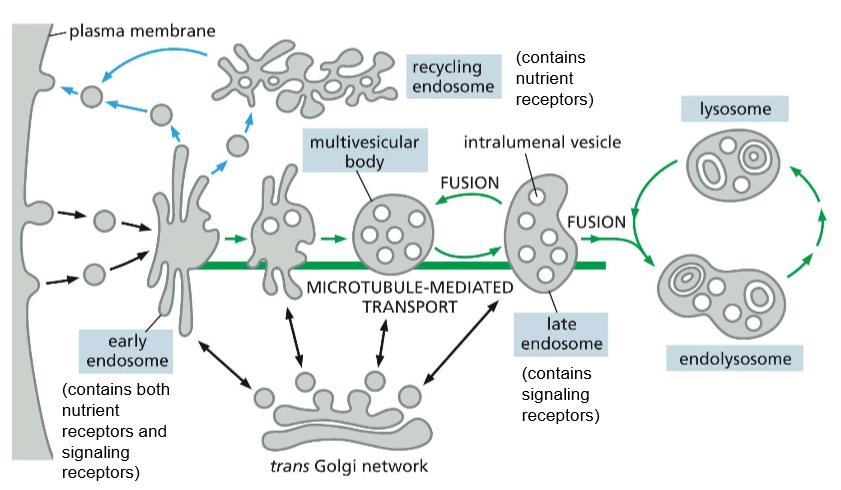
LABELLLLLLLLLLLL
recall that the multivesicular body is still the late endosome!!
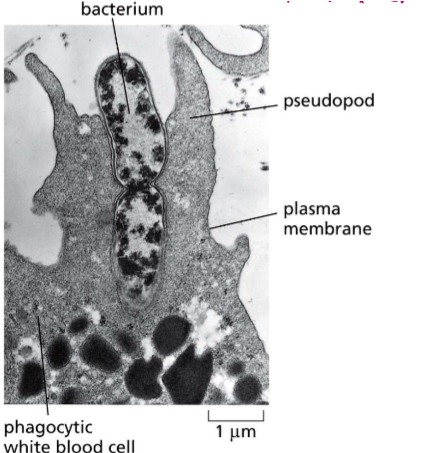
What is phagocytosis?
The intake of particles, molecules, or even other organisms too large for clathrin pits. Actin filaments stretch the cell membrane to ingulf the foreign body
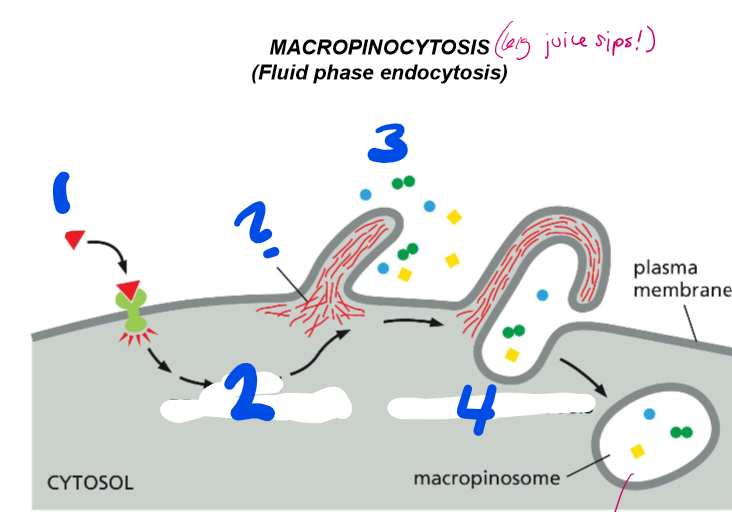
LABELLLLL the steps of macropinocytosis
activation of signaling receptor
Actin rearrangement
Plasma membrane protrusion
vacuole closure
macropinocytosis
? = actin
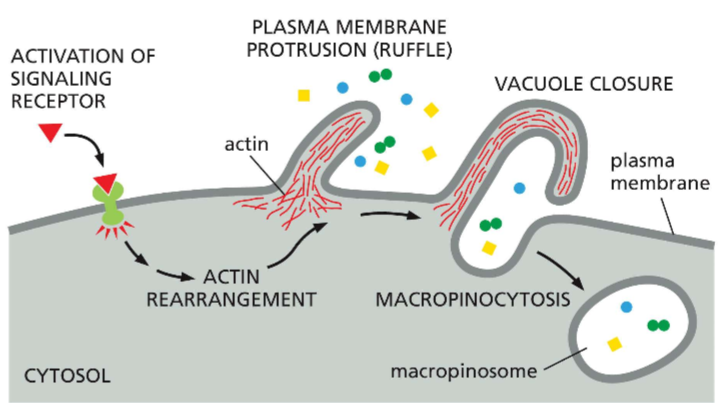
What is macropinocytosis?
Fluid phase endocytosis that is used to capture fluids and and the variety of juice components they may hold
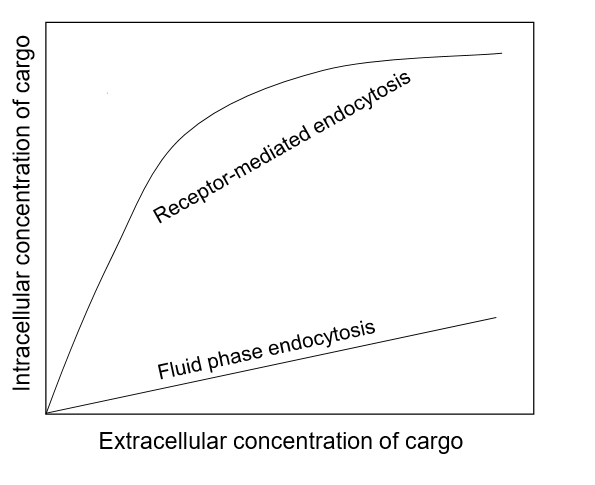
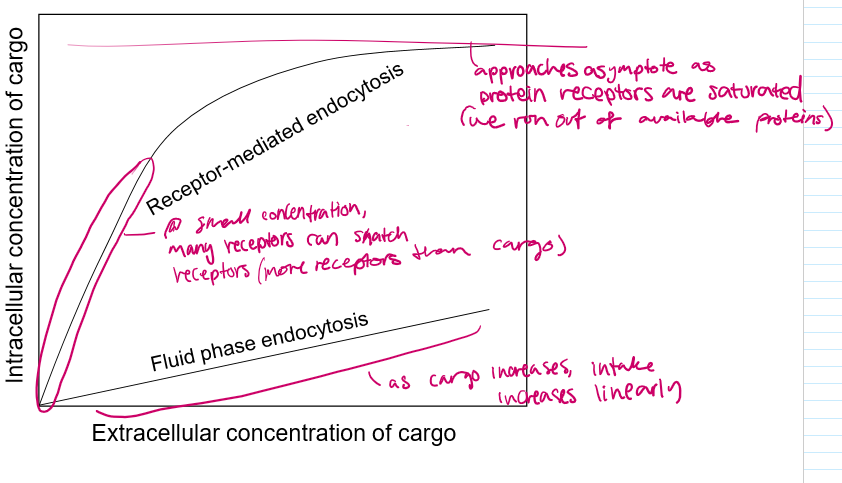
explain the differences in these graphs
For pinocytosis, as the cargo outside increases, the cargo taken in increases linearly
For RM endocytosis, at first it may be a linear increase, but as the extracellular cargo concentration increases, the receptor proteins become saturated and are only able to bring in a constant amount of cargo.