Materials 2
0.0(0)
0.0(0)
Card Sorting
1/110
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
1
New cards
atomic bonding in solid
allows atoms to reduce potential energy state (become more negative)
* more desirable
* more desirable
2
New cards
larger bond energy
stronger the bond
* heat or tensile stress increase interatomic distance and PE
* heat or tensile stress increase interatomic distance and PE
3
New cards
ionic bond
* electrons are transferred
* electrostatic attraction
* full valence shell = reduce PE
* have ions
* electrostatic attraction
* full valence shell = reduce PE
* have ions
4
New cards
covalent bond
* electrons are shared (not 50 50)
* fixed bond lengths and angle
* orbitals overlap
* dipoles
* fixed bond lengths and angle
* orbitals overlap
* dipoles
5
New cards
metallic
* metal bond with another metal regardless of electrons in valence shell
* driving force is need to have full valence shell
* delocalised electron; electron cloud/sea of electron
* electrons are shared
* driving force is need to have full valence shell
* delocalised electron; electron cloud/sea of electron
* electrons are shared
6
New cards
bond type affect conductivity
* metallic bonds allow electron flow bc e- free to travel
* in other bonds, e- not free to travel so insulators
* in other bonds, e- not free to travel so insulators
7
New cards
hydrogen bonds
* molecule has to have hydrogen present
* bond bw water mlc
* electrostatic attraction bw H and O (dipoles)
* weak bond
* bond bw water mlc
* electrostatic attraction bw H and O (dipoles)
* weak bond
8
New cards
bond affect melting point
* heat increase interatomic distance
* larger bond energy, higher melting pt
* E0 = bond energy from peak to x-axis
* E0 = solid = atom vibrate but stay in ‘well’ in solid state
* larger bond energy, higher melting pt
* E0 = bond energy from peak to x-axis
* E0 = solid = atom vibrate but stay in ‘well’ in solid state
9
New cards
polymers in civil and arch engineering
* sealants
* adhesive
* repair and restoration
* interior finishing
* composites
* adhesive
* repair and restoration
* interior finishing
* composites
10
New cards
polymers bonding
* hydrocarbons
* most are H and C; made from fossil fuels
* bonding impact mechanical and thermal properties
* really strong covalent bonds = lot energy to break = high bond energy
* covalent bw carbons; fixed bond length
* covalent bw C and H
* hydrogen bw chains; energy low but multiples across length of mlc
* plastic behavior
* most are H and C; made from fossil fuels
* bonding impact mechanical and thermal properties
* really strong covalent bonds = lot energy to break = high bond energy
* covalent bw carbons; fixed bond length
* covalent bw C and H
* hydrogen bw chains; energy low but multiples across length of mlc
* plastic behavior
11
New cards
chain length
* greater number of carbons, higher mlc weight and the more H bonds
* more condensed state at room temp
* from least to most carbons: gas, liquid, oil, grease, wax, plastic (polymer)
* more condensed state at room temp
* from least to most carbons: gas, liquid, oil, grease, wax, plastic (polymer)
12
New cards
polymer structure
amorphous
* chains are flexible (hydrocarbon chains)
* hydrogen atoms rotate along axis
* result: drained cooked spaghetti w covalent bonds inside each noodle and hydrogen bonds bw noodles ‘sticky’
* chains are flexible (hydrocarbon chains)
* hydrogen atoms rotate along axis
* result: drained cooked spaghetti w covalent bonds inside each noodle and hydrogen bonds bw noodles ‘sticky’
13
New cards
polymer types
* named by smallest repeatable unit = monomer
* R is functional group
* R is functional group
14
New cards
thermoplastics
* linear chains of hydrocarbons
* melt at low temperature
* add functional groups increases yield strength of polymer = amt of stress to get plastic deformation
* decrease percent elongation
* functional group cause friction
* bigger functional group = more friction
* harder for chains to slide + uncoil
* melt at low temperature
* add functional groups increases yield strength of polymer = amt of stress to get plastic deformation
* decrease percent elongation
* functional group cause friction
* bigger functional group = more friction
* harder for chains to slide + uncoil
15
New cards
polyethylene (PE) functional group
R = H
16
New cards
polypropylene (PP) functional group
R = CH3
17
New cards
polyvinylchloride (PVC) functional group
R = Cl
18
New cards
polystyene (PS) functional group
R = C6H5
19
New cards
teflon/PTFE functional group
4R = F
20
New cards
cis-natural rubber latex
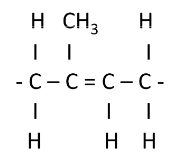
21
New cards
plasticity
* ability to sustain large permanent deformation
* ex groccery bag
* reason: large chains of polymers can uncoil wo breaking primary bonds
* breaking hydrogen bonds bw chains and form new ones
* H-bonds are weak, stress needed to cause permanent deformation low
* ex groccery bag
* reason: large chains of polymers can uncoil wo breaking primary bonds
* breaking hydrogen bonds bw chains and form new ones
* H-bonds are weak, stress needed to cause permanent deformation low
22
New cards
tensile strength polymers
can engineer polymers have different degrees of plastic deformations, ductile or brittle, control by manipulating chemistry
23
New cards
thermal properties polymers
* when melt, covalent bonds stay intact but hydrogen bonds break
* some materials melt from solid to liquid
* thermoplastic have melting temp (Tm) and glass transition temp (Tg)
* below Tg, mvt of chain restricted bc material becomes brittle
* behavior driven by hydrogen bonds
* at extreme high temp scenario, H-bond broken and polymers melt (flow)
* polymers have low melting pt compared to other materials
* some materials melt from solid to liquid
* thermoplastic have melting temp (Tm) and glass transition temp (Tg)
* below Tg, mvt of chain restricted bc material becomes brittle
* behavior driven by hydrogen bonds
* at extreme high temp scenario, H-bond broken and polymers melt (flow)
* polymers have low melting pt compared to other materials
24
New cards
molecular weight polymers
* dep on number of carbons in mlc
* tensile strength increases w mlc weight bc more hydrogen bonds
* levels off as increase MW, so don’t need to max to see optimal strength
* viscosity also increases w decreasing processability
* tensile strength increases w mlc weight bc more hydrogen bonds
* levels off as increase MW, so don’t need to max to see optimal strength
* viscosity also increases w decreasing processability
25
New cards
branches in polymers
* affect density, crystallinity, ductility
* ex. polyethylene - high density
* regions where chanis are aligned = higher density
* more brances = can’t pack together tightly so density lower
* branches have diff composition
* co-polymers and terpolymers
* ex. polyethylene - high density
* regions where chanis are aligned = higher density
* more brances = can’t pack together tightly so density lower
* branches have diff composition
* co-polymers and terpolymers
26
New cards
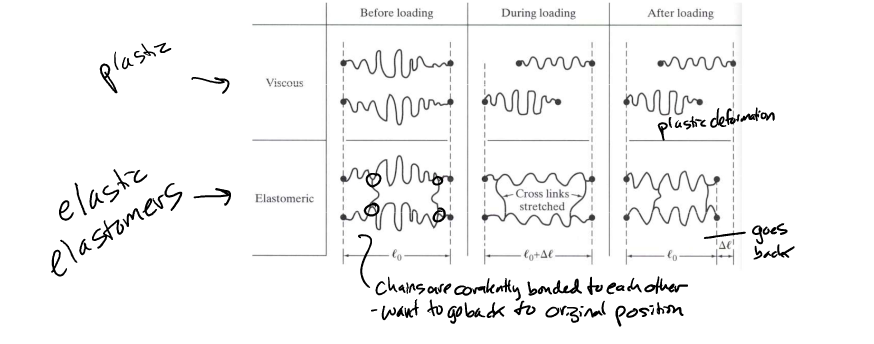
elasticity polymers
certain aspects of polymer composition and structure change deformation properties
* van der waals bonding bw chains
* steric hindrances (large R groups limit flexibility)
* primary chemical bonds bw chains (cross linking)
* van der waals bonding bw chains
* steric hindrances (large R groups limit flexibility)
* primary chemical bonds bw chains (cross linking)
27
New cards
rubber
* is elastomeric
* ‘volcanization’ invented by Goodyear in 1839
* sulfur added at high temps create cross-link
* tech breakthrough
* gummy substance given more rigidity and thermal stability
* latex = thermoplastic; melt the chain; add sulfur for crosslink → need to break cross link to get it to melt so doesn’t melt, it decomposes when heated
* ‘volcanization’ invented by Goodyear in 1839
* sulfur added at high temps create cross-link
* tech breakthrough
* gummy substance given more rigidity and thermal stability
* latex = thermoplastic; melt the chain; add sulfur for crosslink → need to break cross link to get it to melt so doesn’t melt, it decomposes when heated
28
New cards
thermosets
* are not chains
* are hydrocarbons
* have covalent and hydrogen bonds, so heavilty cross-linked called network polymers (like mesh grid)
* brittle and temperature-resistant (higher temp and higher decomposition temp)
* ex: epoxy, silicone, melamine
* negative aspect:
* not fire resistant
* not UV resistant
* thermal response give limited temp range
* are hydrocarbons
* have covalent and hydrogen bonds, so heavilty cross-linked called network polymers (like mesh grid)
* brittle and temperature-resistant (higher temp and higher decomposition temp)
* ex: epoxy, silicone, melamine
* negative aspect:
* not fire resistant
* not UV resistant
* thermal response give limited temp range
29
New cards
wood
naturally occurring polymer composite
* matrix and reinforcement (aligned fibers)
* matrix and reinforcement (aligned fibers)
30
New cards
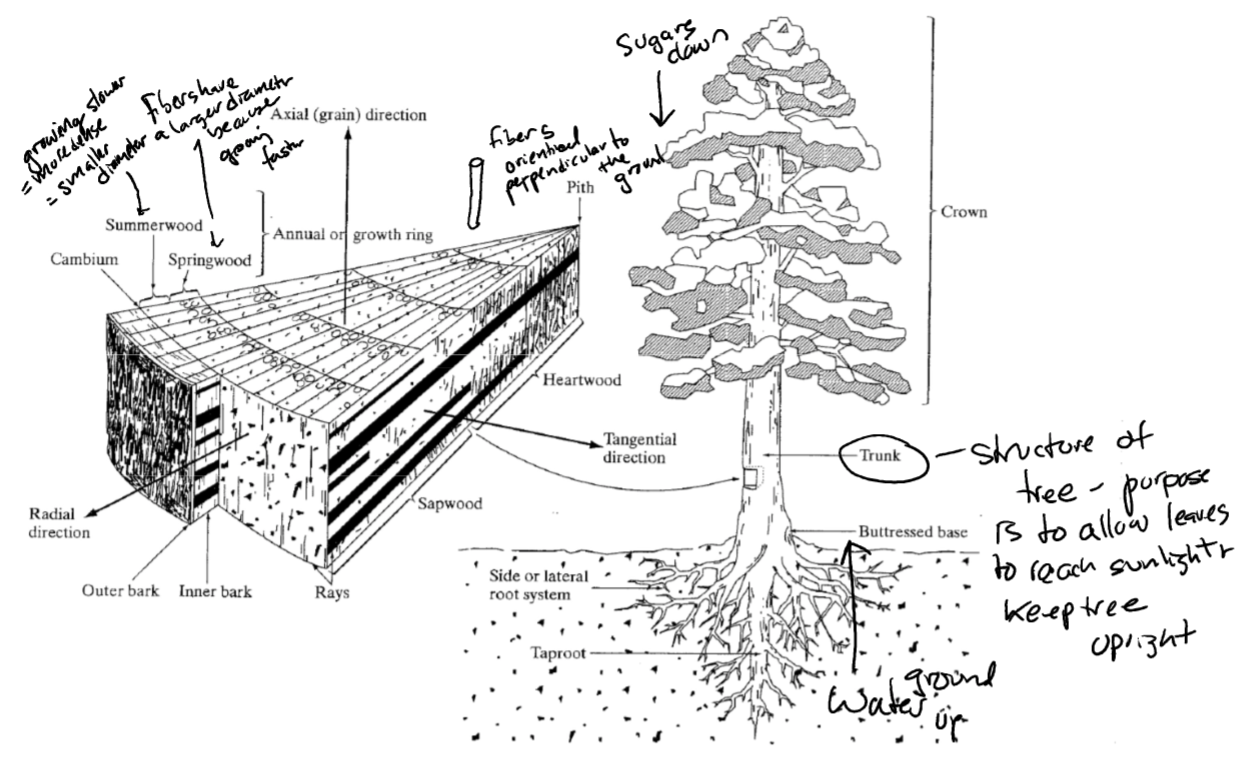
microstructure of wood
* summer wood: grow slower = more dense = smaller diameter
* spring wood: fiber share larger diameter bc growing faster
* fibers orient perpendicular to grain
* water travel from ground up
* trunk: allow leaves to reach sunlight; keep tree upright
* spring wood: fiber share larger diameter bc growing faster
* fibers orient perpendicular to grain
* water travel from ground up
* trunk: allow leaves to reach sunlight; keep tree upright
31
New cards
microstruture wood
* fibers 90% of material
* vertical cells, 2-5 mm, I/d = 100
* rays 10% transverse for transport
* vertical cells, 2-5 mm, I/d = 100
* rays 10% transverse for transport
32
New cards
softwood
most softwoods are soft
* needle like leaves
* can tell spring vs summer wood bc spring less dense and smaller holes
* needle like leaves
* can tell spring vs summer wood bc spring less dense and smaller holes
33
New cards
hardwood
most hardwoods are hard
* have broad leaves
* hardwoods have additional fibers and vessels
* have broad leaves
* hardwoods have additional fibers and vessels
34
New cards
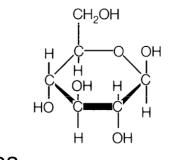
cellulose - aka fiber material
* glucose-based polymer, linear, w thousand units
* covalent bonds in polymer chain
* hydrogen bonding bw chains
* 65-90% crystallinity
* covalent bonds in polymer chain
* hydrogen bonding bw chains
* 65-90% crystallinity
35
New cards
hemicellulose
* polymers made from diff sugar mlc
* hemicellulose and lignins bind cellulose together
* hemicellulose and lignins bind cellulose together
36
New cards
Lignins aka like epoxy in FRP
* 3D structure of phenyl propane units
* provide rigidity
* like network, thermoset polymer = more rigid
* hemicellulose and lignins bind cellulose together
* provide rigidity
* like network, thermoset polymer = more rigid
* hemicellulose and lignins bind cellulose together
37
New cards
anistropic
properties are diff depending on direction of loading
(true of other continuously aligned fiber reinforced composite like FRP > steel reinforced concrete)
(true of other continuously aligned fiber reinforced composite like FRP > steel reinforced concrete)
38
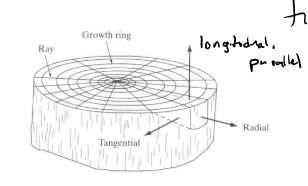
New cards
how is wood strongest
wood strongest in parallel to grain under compressive load
* mainly wood strongest under parallel to grain direction (longitudinal)
* perpendicular to grain = radial and tangential
* mainly wood strongest under parallel to grain direction (longitudinal)
* perpendicular to grain = radial and tangential
39
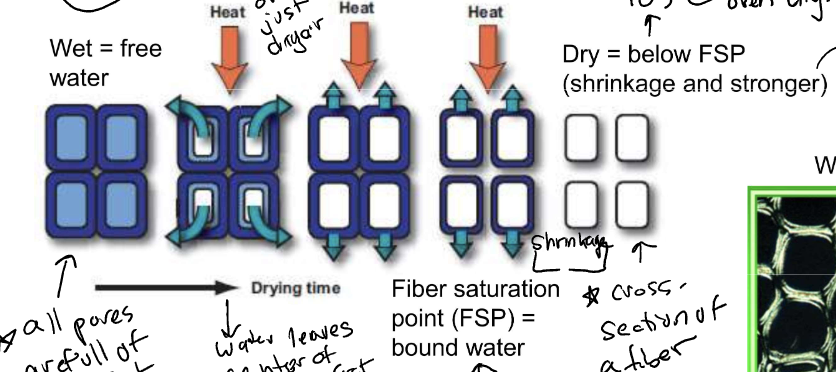
New cards
moisture in wood
* wet = free water
* all pores full of water and surface water
* water leaves center of fiber first
* fiber saturation point (FSP) = bound water
* center of water has evaporate but water on wall still
* Dry = below FSP
* shrink and become stronger, denser (over 105°C)
* all pores full of water and surface water
* water leaves center of fiber first
* fiber saturation point (FSP) = bound water
* center of water has evaporate but water on wall still
* Dry = below FSP
* shrink and become stronger, denser (over 105°C)
40
New cards
shrinkage of wood
* towards the edge more warping, in the middle less warping
* shrinking edge-grain wood or radial to growth rings
* mainly shrinking of flat-grain wood or tangential to growth ring
* shrink lengthwise very small (2% of radial shrinkage)
* shrinking edge-grain wood or radial to growth rings
* mainly shrinking of flat-grain wood or tangential to growth ring
* shrink lengthwise very small (2% of radial shrinkage)
41
New cards
flaws in wood
* lumber/wood have many flaw; wood are small specimen that are flaw free for testing
* flaws affect mech properties
* flaws affect mech properties
42
New cards
knot flaw
where branches where
43
New cards
check flaw
like a crack in wood
44
New cards
pros and cons of wood
pros:
* pretty
* sustainable
* lightweight (strength to weight ratio)
Cons:
* swelling
* mold, rot bc moisture
* size limited
* anisotropy
engineers solve these problems by creating engineered wood
* pretty
* sustainable
* lightweight (strength to weight ratio)
Cons:
* swelling
* mold, rot bc moisture
* size limited
* anisotropy
engineers solve these problems by creating engineered wood
45
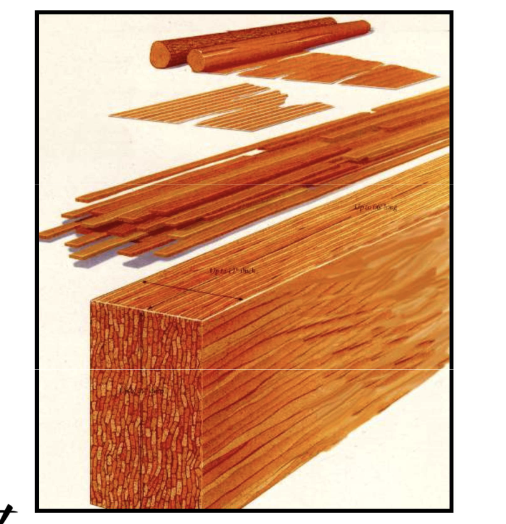
New cards
engineered panel products
* plywood: flaws only ply thick, allow bigger pieces
* waferboard
* composite
* structural particleboard: small pieces glued together; less waste, less anistropyl; flaw not important
* oriented strand board
* waferboard
* composite
* structural particleboard: small pieces glued together; less waste, less anistropyl; flaw not important
* oriented strand board
46
New cards
laminated veneer lumber
like shaving a tree and removing its circumference surface area

47
New cards
parallel strand lumber
cutting parallel strips from lumber
48
New cards
why use engineered lumber products
* consistency of products
* reduce swelling/shrinkage problems
* engineered lumber 6-8% moisture content from factory
* no warp, twist, crook, checking, wane
* COV < 11%
* design capabilities
* increased strength and stiffness
* environmental issues & availability
* reduced availability of old-growth timber
* more efficient use of wood fiber
* use under-valued species
* reduce swelling/shrinkage problems
* engineered lumber 6-8% moisture content from factory
* no warp, twist, crook, checking, wane
* COV < 11%
* design capabilities
* increased strength and stiffness
* environmental issues & availability
* reduced availability of old-growth timber
* more efficient use of wood fiber
* use under-valued species
49
New cards
SP #2
southern pine
50
New cards
rubber bridge bearings
bearing must resist:
* compressive forces from weight of bridge
* shear forces as bridge shortens and lengthens due to thermal expansion and contraction
* need steel plates bc reduce deformation under compression for more stability
* low maintenance alternative
* (before had mechanical steel bering w bolt and needed a lot of maintenance)
* compressive forces from weight of bridge
* shear forces as bridge shortens and lengthens due to thermal expansion and contraction
* need steel plates bc reduce deformation under compression for more stability
* low maintenance alternative
* (before had mechanical steel bering w bolt and needed a lot of maintenance)
51
New cards
based isolation bearings
reduce shaking of building during earthquake
52
New cards
Fiber reinforced polymer (FRP)
* fiber reinforced = reinforcement; polymer = matrix
* used to wrap damaged structural elements
* wrap elements in seismic regions
* used as substitute for steel-reinforcing bar
* used to wrap damaged structural elements
* wrap elements in seismic regions
* used as substitute for steel-reinforcing bar
53
New cards
fiber reinforced (reinforcement)
* carbon
* e-glass
* aramid (kevlar)
* fiber strong - carry load
* fiber continuous and aligned (parallel)
* e-glass
* aramid (kevlar)
* fiber strong - carry load
* fiber continuous and aligned (parallel)
54
New cards
polymer as a matrix
* epoxy
* vinly ester
* polyesters
* polymers encase and protected; allow load transfer bw fibers
* vinly ester
* polyesters
* polymers encase and protected; allow load transfer bw fibers
55
New cards
CFRP
carbon + epoxy
56
New cards
GFRP
e-glass + vinyl ester
57
New cards
AFRP
aramid + polyesters
58
New cards
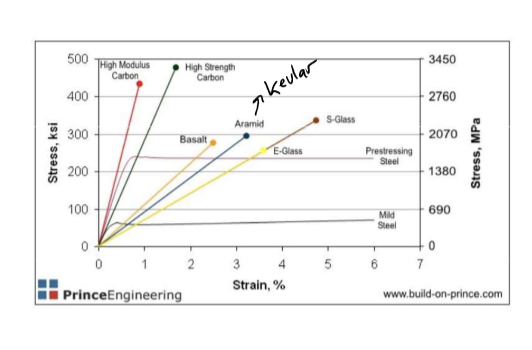
FRP stress vs strain
* fiber strong brittle, high E
* resin lopw E, ductile, weak E
* FRP composite in bewteen
* resin lopw E, ductile, weak E
* FRP composite in bewteen
59
New cards
forms of FRP
* rods
* shapes
* steel or FRP
* bars
* typically made from steel, but can be made from FRP bc doesn’t rust
* planks/strips
* shapes
* steel or FRP
* bars
* typically made from steel, but can be made from FRP bc doesn’t rust
* planks/strips
60
New cards
dry fabrics
* structural repair and strengthening
* flexible and easy to be installed (by wet lay-up or dry lay-up) to fit geometry of members
* workable in areas w limited space/access
* thin profile meet desirable asthetics
* flexible and easy to be installed (by wet lay-up or dry lay-up) to fit geometry of members
* workable in areas w limited space/access
* thin profile meet desirable asthetics
61
New cards
flexural strengthening FRP
* transverse straps for enhancing bonding and anchorage
* fiber’s orientation must be parallel to beam
* tensile strengthening along bottom of beam
* uses CFRP
* fiber’s orientation must be parallel to beam
* tensile strengthening along bottom of beam
* uses CFRP
62
New cards
confinement FRP
* fiber’s orientation horizontally around column
* in compression, poisson’s ratio causes middle to expand out
* FRP used to confine column for indirectly increasing compressive strength by putting column in triaxial stress
* in compression, poisson’s ratio causes middle to expand out
* FRP used to confine column for indirectly increasing compressive strength by putting column in triaxial stress
63
New cards
examples of FRP use
* fire damage repairment
* installed steel wire mesh for securing bonding
* polyethylene tubing installed for injecting epoxy to seal cracks
* cap and girders repairement: use fresh concrete to mimic shape of original member
* longitudinal and “U” wrap CFRP installed on girders per design
* installed steel wire mesh for securing bonding
* polyethylene tubing installed for injecting epoxy to seal cracks
* cap and girders repairement: use fresh concrete to mimic shape of original member
* longitudinal and “U” wrap CFRP installed on girders per design
64
New cards
asphalt
polymer composite
* asphalt is matrix
* aggregates are reinforcements (same as concrete)
* asphalt is matrix
* aggregates are reinforcements (same as concrete)
65
New cards
asphalt’s other name
* hot mix asphalt (HMA)
* blacktop
* tarmac
* Macadam
* plant mix
* bituminous material
* blacktop
* tarmac
* Macadam
* plant mix
* bituminous material
66
New cards
what is asphalt
* dark brown to black cementitious (adhesive) material where bitumens occur in nature or obtained in petroleum processing
* asphalt concrete = asphalt binder + 95% vol mineral aggregates
* asphalt concrete = asphalt binder + 95% vol mineral aggregates
67
New cards
how many paved roads in US are asphalt
800,000 roads (around 18 billion tons of asphalt)
68
New cards
reusability of asphalt
most reused product in america
* can add recycled tires, engine oil, slags, glass, shingles
* can add recycled tires, engine oil, slags, glass, shingles
69
New cards
asphalt history
first use in US in 1870
* use naturally occuring asphalt from surface of lake on island of Trinidad
1900s: petroleum asphalt develop
* waste product from refining of crude oil
* bottom of barrel
* use naturally occuring asphalt from surface of lake on island of Trinidad
1900s: petroleum asphalt develop
* waste product from refining of crude oil
* bottom of barrel
70
New cards
components of asphalt
* high mlc weight hydrocarbons
* long hydrocarbon chains = high mlc weight
* asphaltenes
* resins
* oils
* depending on proportions, mech properties of asphalt differ
* long hydrocarbon chains = high mlc weight
* asphaltenes
* resins
* oils
* depending on proportions, mech properties of asphalt differ
71
New cards
asphaltenes
* large, discrete solid inclusions (black)
* high viscocity
* highest mlc weight
* solid at room temp
* fewer = softer; more = harder
* high viscocity
* highest mlc weight
* solid at room temp
* fewer = softer; more = harder
72
New cards
resins asphalt
* semi-solid/solid at room temp
* fluid when heated; brittle when cold
* lower mlc weight than asphaltene
* fluid when heated; brittle when cold
* lower mlc weight than asphaltene
73
New cards
oil asphalt
* colorless liquid
* soluble in most solvent
* allow asphalt to flow
* soluble in most solvent
* allow asphalt to flow
74
New cards
asphalt uses
* pavements - have layers
* asphalt surface → granular base and subase (aggregates) → prepared subgrade
* sealing
* waterproofing
* repair
* asphalt surface → granular base and subase (aggregates) → prepared subgrade
* sealing
* waterproofing
* repair
75
New cards
making pavements
asphalt cement and agg mixed together at high temp and compacted on road while still hot
* fluid at high temp bc hydrogen bonds bw mlc break causing polymer to melt
* fluid at high temp bc hydrogen bonds bw mlc break causing polymer to melt
76
New cards
mix design pavement
addresses performace of asphalt under working conditions
* dep on
* climate (hot vs cold); too cold brittle; too hot flow
* loading (traffic)
* time (oxidation, moisture)
* dep on
* climate (hot vs cold); too cold brittle; too hot flow
* loading (traffic)
* time (oxidation, moisture)
77
New cards
hauling asphalt
tandem axle end dump truck
* hot asphalt + agg mixture
have paver at the back ususally
* hot asphalt + agg mixture
have paver at the back ususally
78
New cards
placement asphalt
self propelled paver
79
New cards
compacting asphalt pavement
vibratory roller
* prevent further compaction
* provide shear strength or resistance to rutting
* ensure mixture is waterproof
* prevent excessive oxidation of asphalt binder
* prevent further compaction
* provide shear strength or resistance to rutting
* ensure mixture is waterproof
* prevent excessive oxidation of asphalt binder
80
New cards
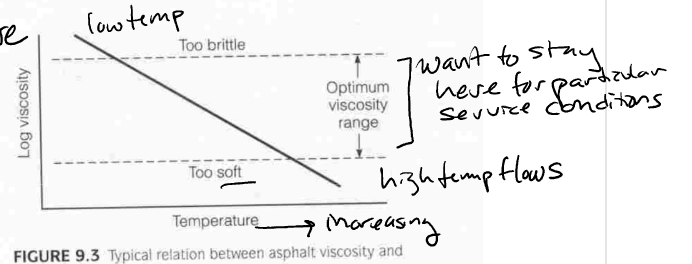
climate design criteria for pavements
* behavior temp dependent like thermoplastic polymer
* when heated
* asphaltenes dissolve in resins
* resins dissolve in oils
* less viscous (flow more)
* when cooled = opposite
* choose correct type of binder for climate
* hot climates: use harder grade to avoid rutting
* cold climates: use softer grades to avoid temp cracking
* when heated
* asphaltenes dissolve in resins
* resins dissolve in oils
* less viscous (flow more)
* when cooled = opposite
* choose correct type of binder for climate
* hot climates: use harder grade to avoid rutting
* cold climates: use softer grades to avoid temp cracking
81
New cards
loading design criteria pavement
function of repeated traffic loads over time in wheel paths create fatigue cracking
82
New cards
time design criteria asphalt
* aging/oxidation and moisture damage occur over time
* can lead to rutting, cracking, and agg debonding
* can lead to rutting, cracking, and agg debonding
83
New cards
other design criteria for pavements
* resistance to skidding (texture): test agg for abrasion resistance
* sufficient workability for mixing, placing, compaction
* cost and availability
* sufficient workability for mixing, placing, compaction
* cost and availability
84
New cards
SUPERPAVE
superior performing asphalt pavements: designed in 1987
* is performance-based
* choose asphalt binder **NOT** on components **BUT** on potential for rutting, fatigue cracking, thermal cracking (based on climate, load, time)
* have trade offs: binders good load resistance = low temp cracking
* is performance-based
* choose asphalt binder **NOT** on components **BUT** on potential for rutting, fatigue cracking, thermal cracking (based on climate, load, time)
* have trade offs: binders good load resistance = low temp cracking
85
New cards
SUPERPAVE concrete design
asphalt binder grade determined based on performance in lab
* bulk specific gravitiy
* shear
* tensile strength and fracture
* creep
* bending beam rheometer
* bulk specific gravitiy
* shear
* tensile strength and fracture
* creep
* bending beam rheometer
86
New cards
asphalt binder grading
performance grading “PG X-Y”
* X and Y max and min designing temps
* ex PG 52-28 => temp 52 to -28°C
* second number always negative
* X and Y max and min designing temps
* ex PG 52-28 => temp 52 to -28°C
* second number always negative
87
New cards
surface recycling
* more like reusing
* heat top 25 mm of pavement
* repair minor cracks and roughness
* heat top 25 mm of pavement
* repair minor cracks and roughness
88
New cards
central plant recycling
mill old pavement to get reclaimed asphalt pavement (RAP) and create new HMA
89
New cards
in place recycling
rip and pulverize old pavement - add new agg, water, asphalt emulsion - mix, grade, compact
90
New cards
add rubber tires to asphalt concrete
* increase elasticity and stiffness
* increase cement-agg bond
* reduce thermal cracking and freeze-thaw damange
* added w binder or w aggregate
* increase cement-agg bond
* reduce thermal cracking and freeze-thaw damange
* added w binder or w aggregate
91
New cards
metals in tension in elastic region
have elastic deformation bc stretching bonds bw atoms and when let go, atoms go back to preferred position which is min bond length and energy
92
New cards
crystal
have regular, repeating patterns of atoms
93
New cards
amorphous
shapeless; random/semi-random arrangements of atoms
94
New cards
unit cell
repeated to make a pattern
* want close packing bc limited geometries for atoms of a single size to pack as closely as possible
* want close packing bc limited geometries for atoms of a single size to pack as closely as possible
95
New cards
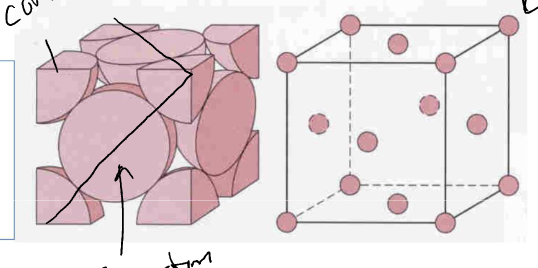
Face Centered Cubic (FCC)
* atom on each corner of cube and atom centered on each face at cube
* packing in close-packed plane
* all optimal bond distance
* line through face diagonals doesn’t go through empty space
* packing in close-packed plane
* all optimal bond distance
* line through face diagonals doesn’t go through empty space
96
New cards
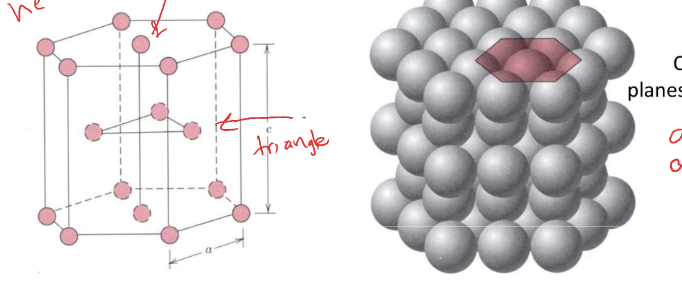
hexagonal close packed (HCP)
* packing in closed packed plane
* hexagonal ring with face centered and triangle in middle
* alternating layers of those packed plane
* hexagonal ring with face centered and triangle in middle
* alternating layers of those packed plane
97
New cards
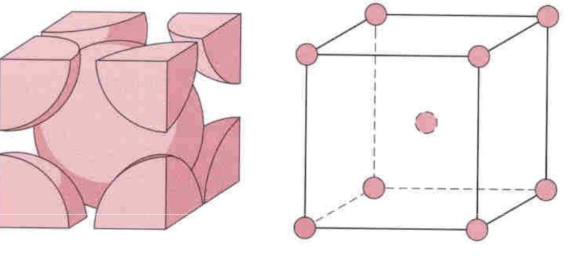
Body Centered Cubic (BCC)
* atoms on each corner of cube and one in center of body
* closely packed planes intersect diagonally
* closely packed planes intersect diagonally
98
New cards
defects in crystals
* they exist (almost all crystals have defect)
* exist bc of entropy (chaos)
* interstitials and vacancies happen spontaneously in all crystals;
* can control substitutions and manipulate them to achieve properties of material
* exist bc of entropy (chaos)
* interstitials and vacancies happen spontaneously in all crystals;
* can control substitutions and manipulate them to achieve properties of material
99
New cards
1-D imperfection
simple cubic unit cell crystal
* point defects
* in region of defect, surrounding atoms are not at idea bond length, so not at lowest energy state
* point defects
* in region of defect, surrounding atoms are not at idea bond length, so not at lowest energy state
100
New cards
vacancy
single missing atom
* surrounding atoms pull toward empty space
* surrounding atoms pull toward empty space