week 4 - intestines
1/181
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
182 Terms
describe the small intestine
•It extends from the pyloric orifice of the stomach to ileocecal junction where the ileum joins the cecum (first part of the large intestine)
•It is divided into:
•Duodenum
•Jejunum
•Ileum
explain the location of the small intrestine
•Proximal 2/5th is the jejunum and most of this section is the Left Upper Quadrant
•Distal 3/5th is the ileum and most of this is in the Right Lower Quadrant
•No clear division of the jejunum and ileum but they have distinctive characteristics
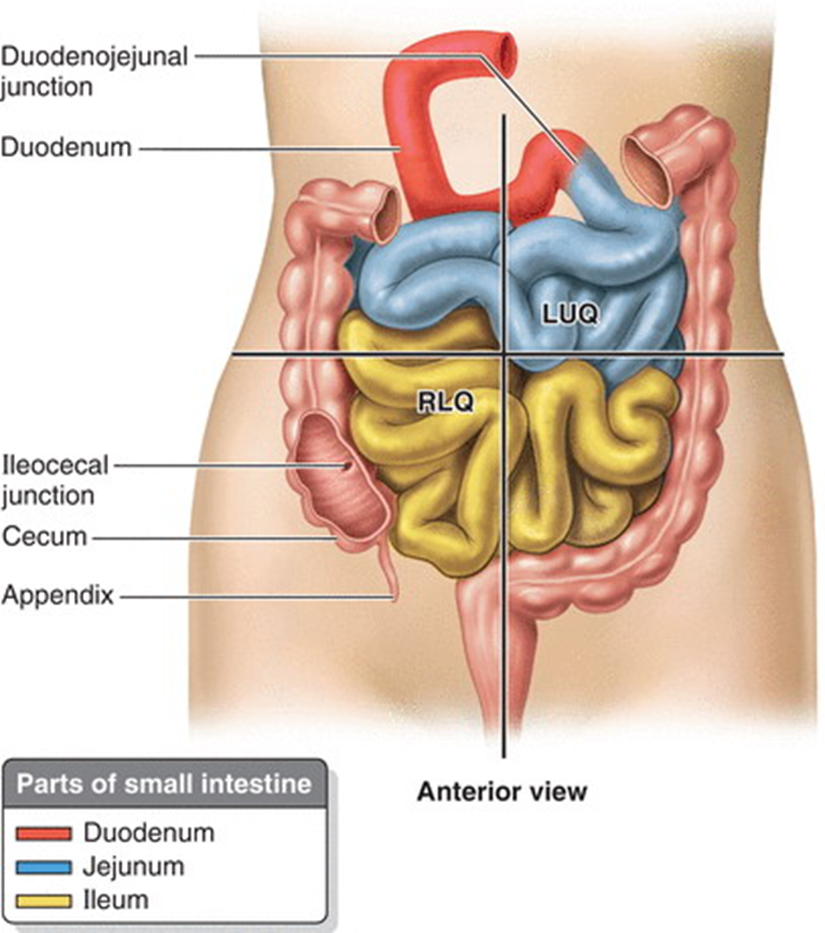
distinguish between the jejunum and ileum
Jejunum | Ileum |
Larger diameter | Smaller diameter |
Thicker wall | Thinner walls |
Less mesenteric fat | More mesenteric fat |
More plicae circulares | Fewer/ less prominent plicae circulares |
Less prominent arterial arcades | More arterial arcades |
Longer Vasa Recta (Straight Arteries) | Shorter Vasa Recta |
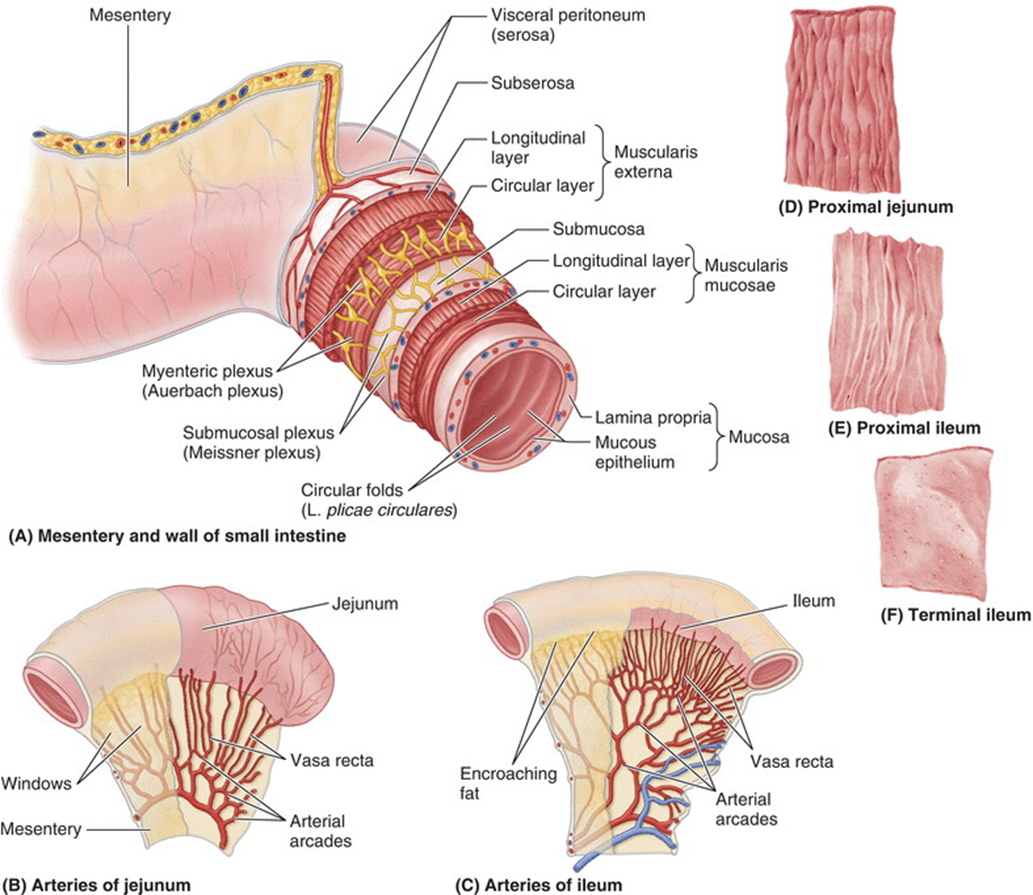
explain the blood supply of the jejunum and ileum
•Jejunal and ileal arteries
•From superior mesenteric artery
•Approx 15-18 branches that unite to form arterial arcades

•These travel within the mesentery of the small intestine
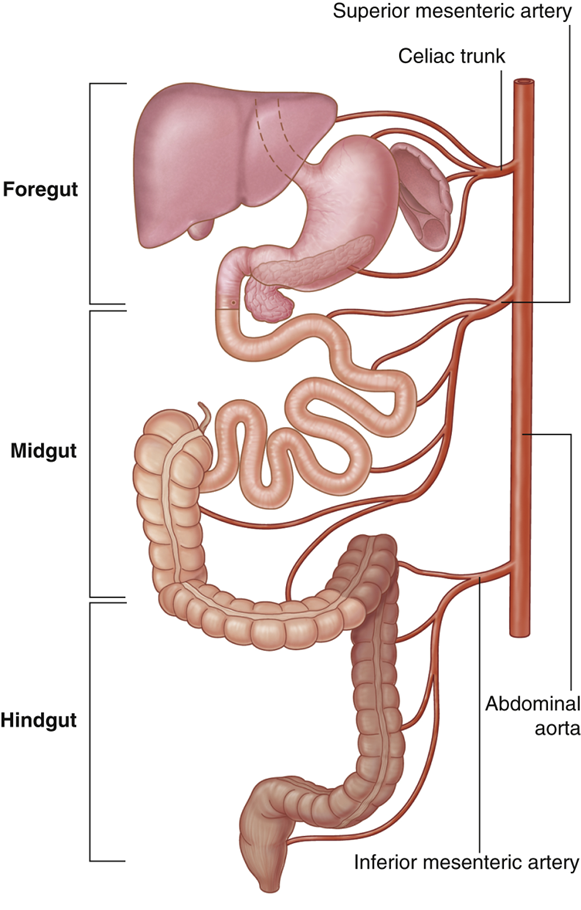
explain the superior mesenteric artery blood supply
•SMA from the abdominal aorta at L1 vertebra (inferior to the celiac trunk)
•Supplies blood to the structures that formed from the midgut
•Midgut develops into the distal part of the duodenum, jejunum, ileum, ascending colon and proximal two-thirds of the transverse colon

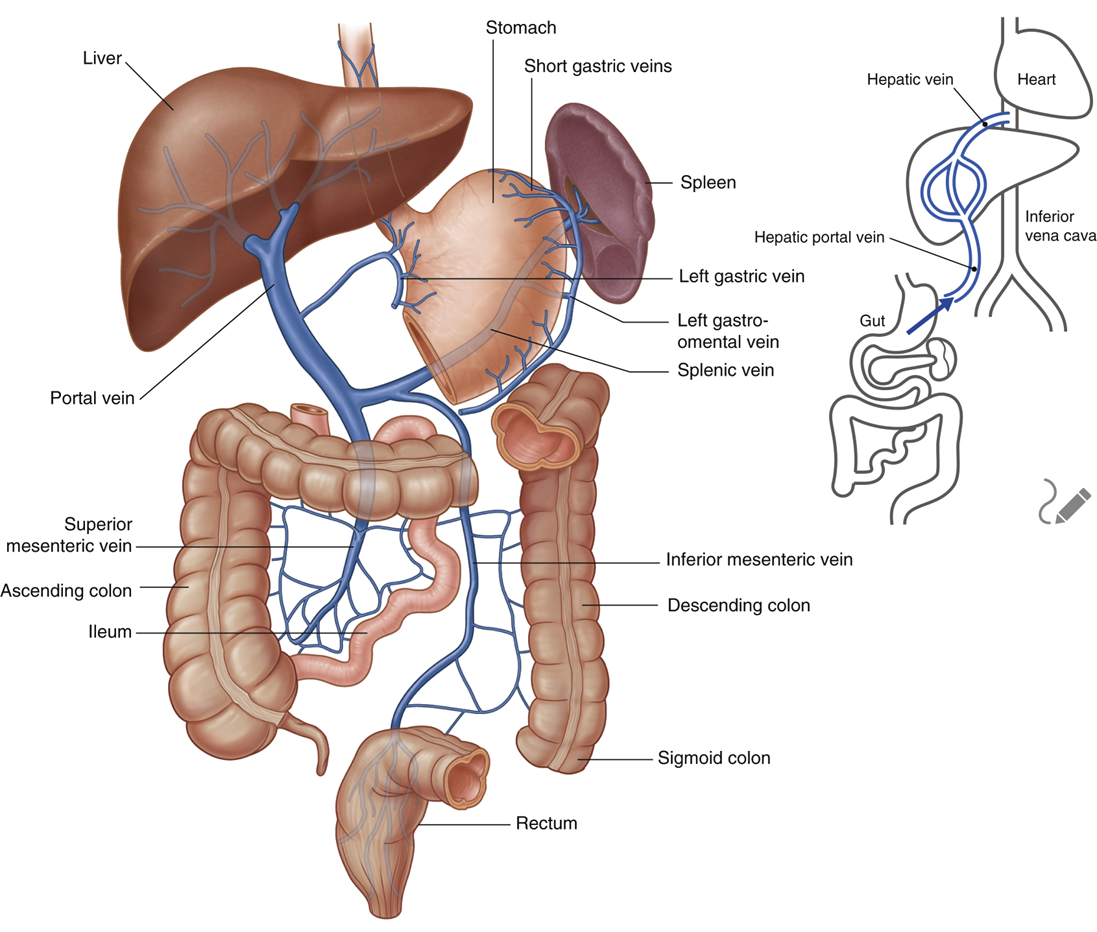
explain the venous drainage via the superior mesenteric vein
•Superior Mesenteric Vein drains the jejunum and ileum
•It begins in the right iliac fossa (right groin) and ascends in the mesentery to the right of the superior mesenteric artery
•It joins with the splenic vein, posterior to the neck of pancreas, to form the hepatic portal vein
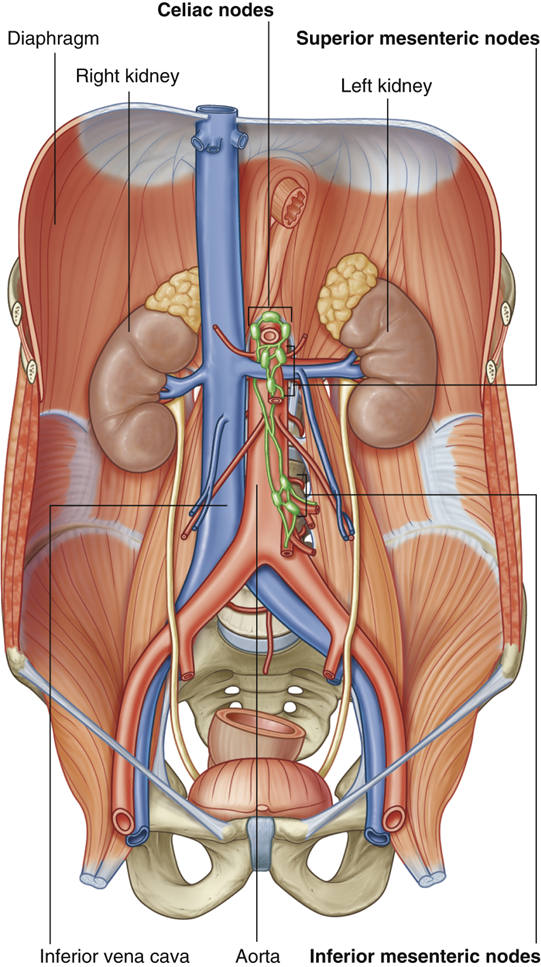
explain the lymphatic drainage of the small intestine
•Small intestine has lacteals within the villi of its walls:
•Specialized lymphatic vessels that absorb emulsified fats
Drains to pre-aortic lymph nodes:
•Celiac nodes (derivatives of the foregut)
•Superior mesenteric nodes (derivatives of the midgut)
•Inferior mesenteric nodes (derivatives of the hindgut)
•Inferior mesenteric nodes drain to superior mesenteric nodes which drain to celiac nodes
•Celiac nodes eventually drain to thoracic duct that drains into the venous system at the left subclavian/ brachiocephalic vein
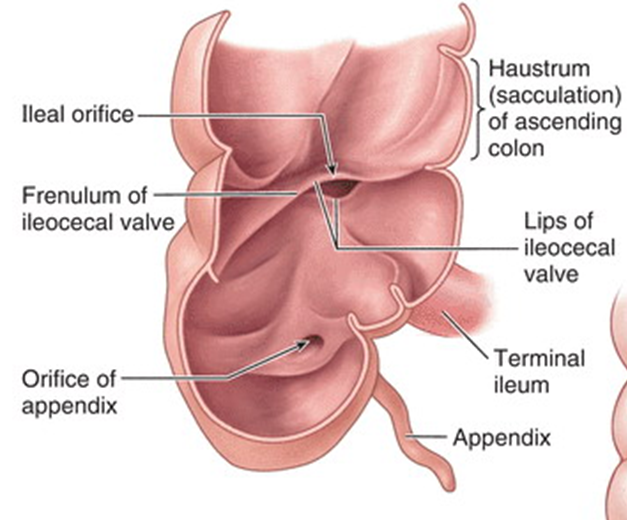
describe the ileocecal junction
•Small intestine ends at the ileocecal junction
•Ileum opens into the large intestine, where the cecum and ascending colon join
•Two flaps projecting into the lumen of the large intestine (the ileocecal fold ) surround the opening
describe Meckel’s (Ileal) Diverticulum ( NOT an ILO)
•Common congenital disorders of digestive system
•Remnant of the proximal yolk stalk
•Can be asymptomatic but can cause symptoms like haemorrhaging or bowel obstruction in others
•Inflammation of diverticulum can mimic pain produced by appendicitis
describe the large intestine
•Main site of water absorption from chyme, resulting in formation of faeces, which can be stored until defecation occurs
Made up of:
•Cecum
•Colon
•Rectum
•Anal canal
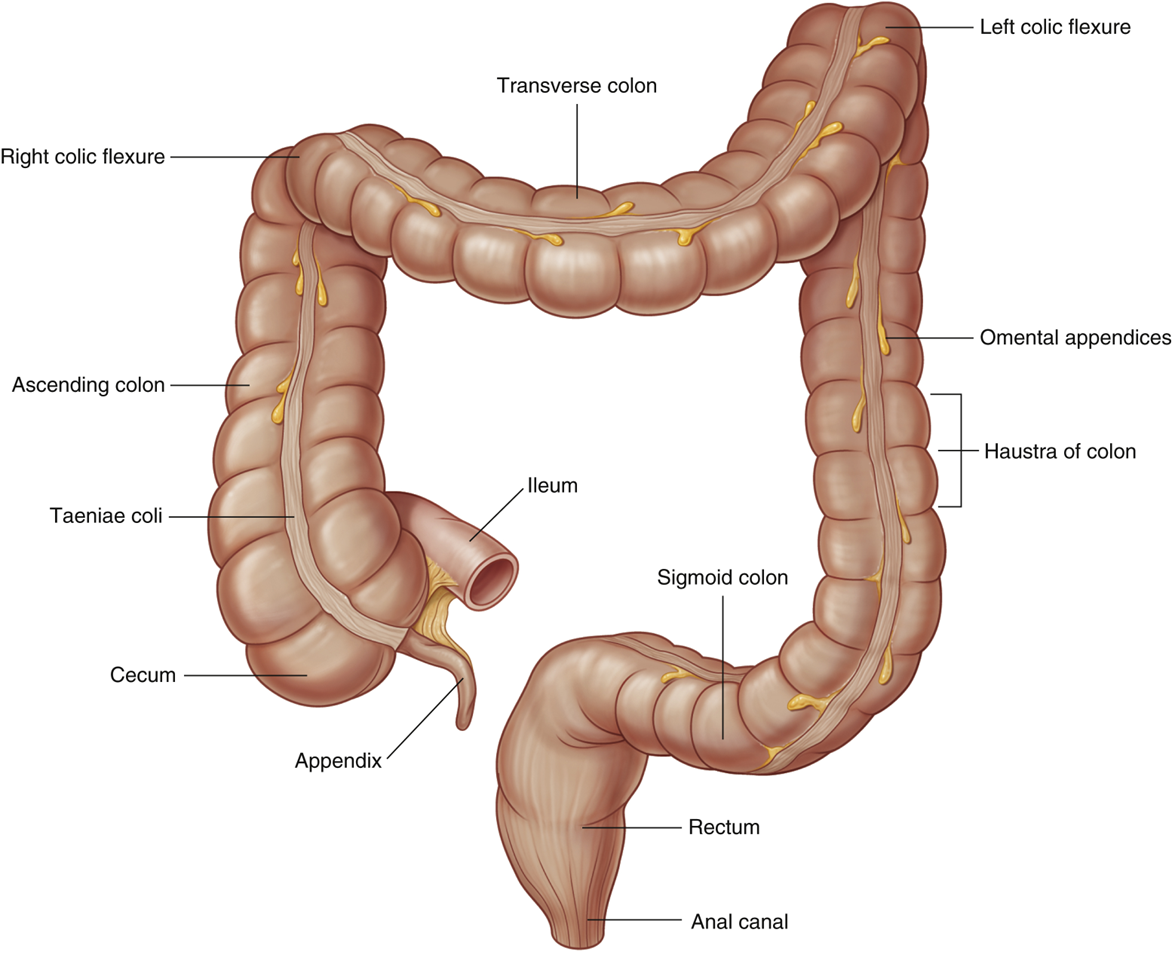
describe features of the large intestine
•Omental (epiploic) appendices
•Small, fatty projections
•Absent on rectum
•Teniae coli
•Longitudinal muscles primarily observed in the cecum and colon
•Shorter than large intestine so cause haustra
•Haustra
•Sacculations of the wall of the colon
•Larger diameter than small intestine
describe the cecum and appendix
The cecum is the first part of the large intestine (in the right groin)
•Inferior to the ileocecal opening
•continuous with the ascending colon
The appendix is a narrow, hollow, tube connected to the cecum
•Continuous longitudinal muscular coat
•Teniae coli converge
•Thought to be important for immune system and gut health
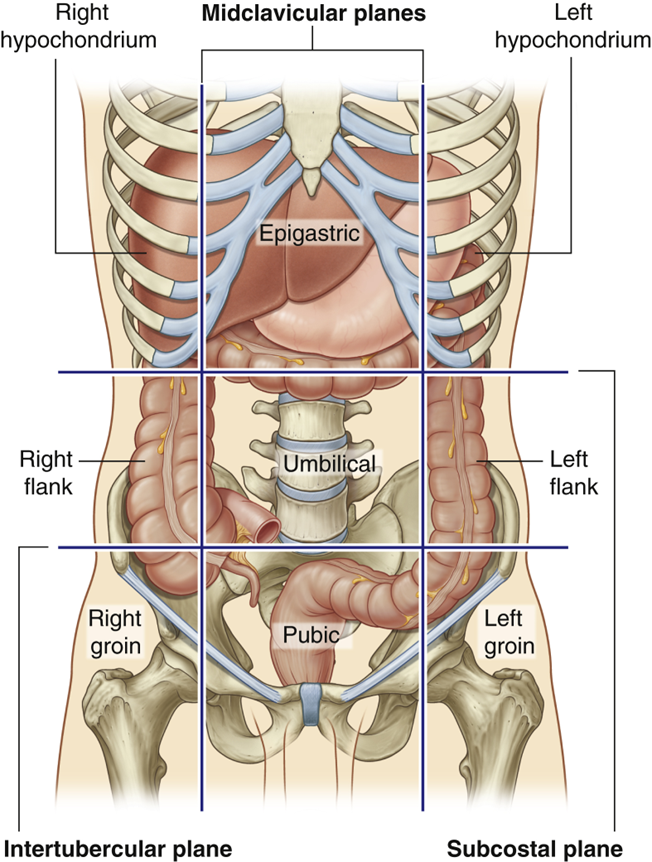
describe the colon
•Ascending colon (right flank)
Right Colic (hepatic) Flexure (right hypochondrium)
•Transverse colon (*can vary)
Left Colic (Splenic) Flexure (left hypochondrium)
•Descending Colon (left flank)
•Sigmoid Colon (pubic)
describe the rectum
•Rectum pelvic part of the digestive tract and extends from the sigmoid colon
•Although named due to it being the straight part of the large intestine it does have flexures:
•Ant-Post so S-shape when viewed laterally
•Sacral flexure
•Anorectal flexure
•Lateral Flexures at transverse folds
•Superior, inferior & intermediate
•Rectal ampulla is final part of rectum and can expand to hold faecal mass temporarily until it is expelled

describe the anal canal
•The anal canal extends from ampulla of the rectum to the anus (external outlet of the GI tract)
Has internal and external sphincters:
Internal sphincter which is involuntary
•Superior two thirds usually contracted but relaxes in response to distention of ampulla
•This then requires voluntary contraction of the:
External, which is voluntary (striatal muscle) and is relaxed to allow defecation to occur (involvement of puborectalis, part of pelvic diaphragm)
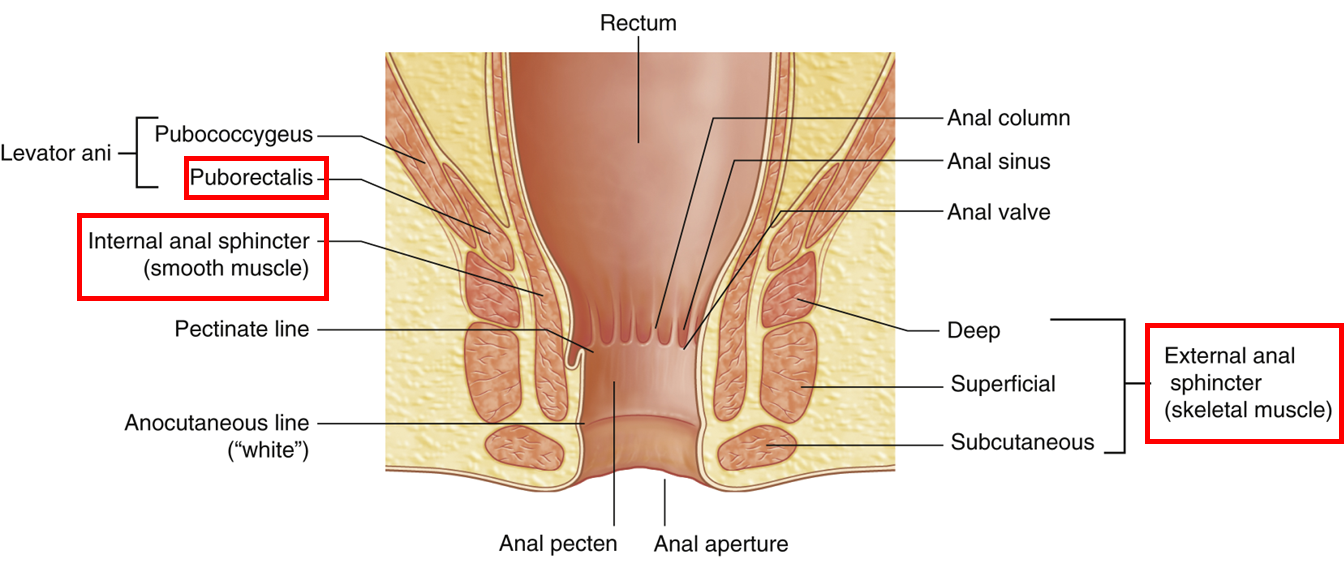
describe the different regions of the anal canal
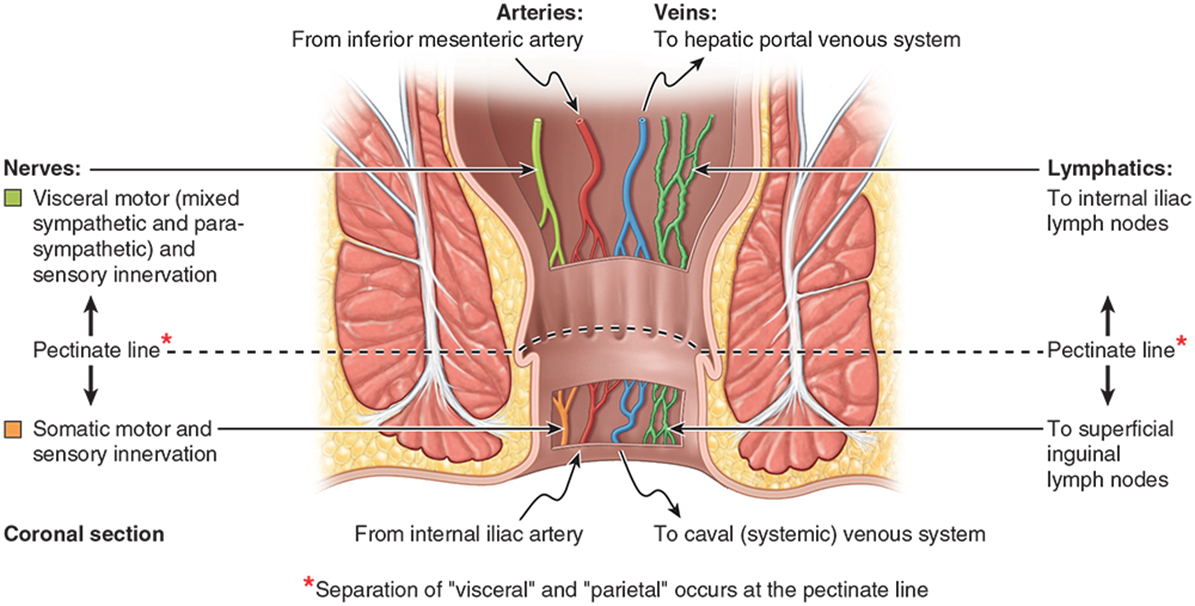
Superior half anal canal has anal columns (between which are anal sinuses) which are joined inferiorly
•This creates an irregular line known as the pectinate line
•Approximate position of the cloacal membrane in the fetus (above from endoderm, below from ectoderm)
•Different blood supply, drainage and innervation
Inferior half of canal is anal pecten, bordered inferiorly by the anocutaneous line where the coverings become skin (stratified squamous epithelium)
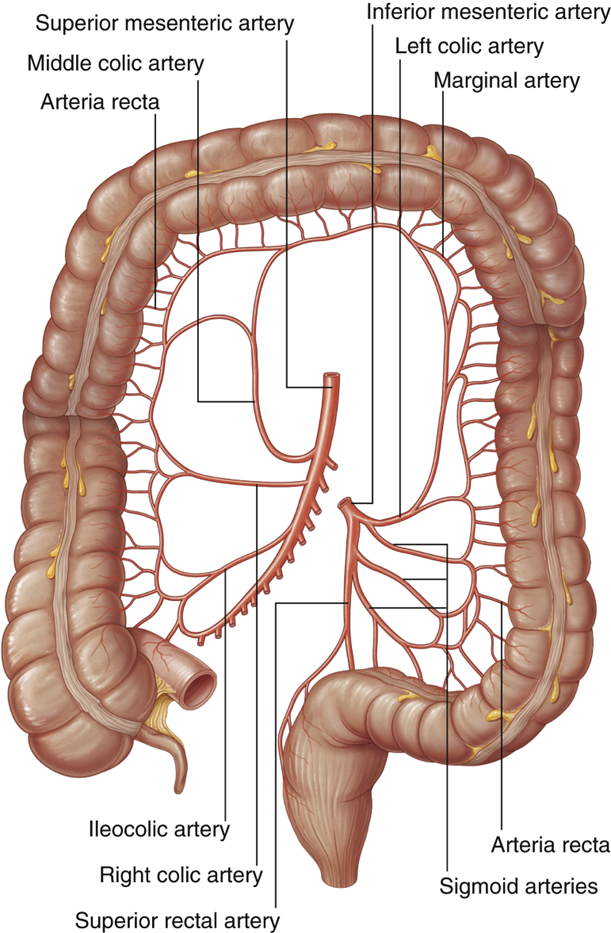
describe the blood supply of the colon
•Superior Mesenteric Artery
•Ileocolic Artery
•Right Colic Artery
•Middle Colic Artery
•Inferior Mesenteric Artery
•Left Colic Artery
•Sigmoid Arteries
•Superior rectal Artery
•Distal 1/3 of transverse colon
•Descending colon
•Sigmoid colon
•Superior part of the rectum
SMA & IMA connected by marginal artery
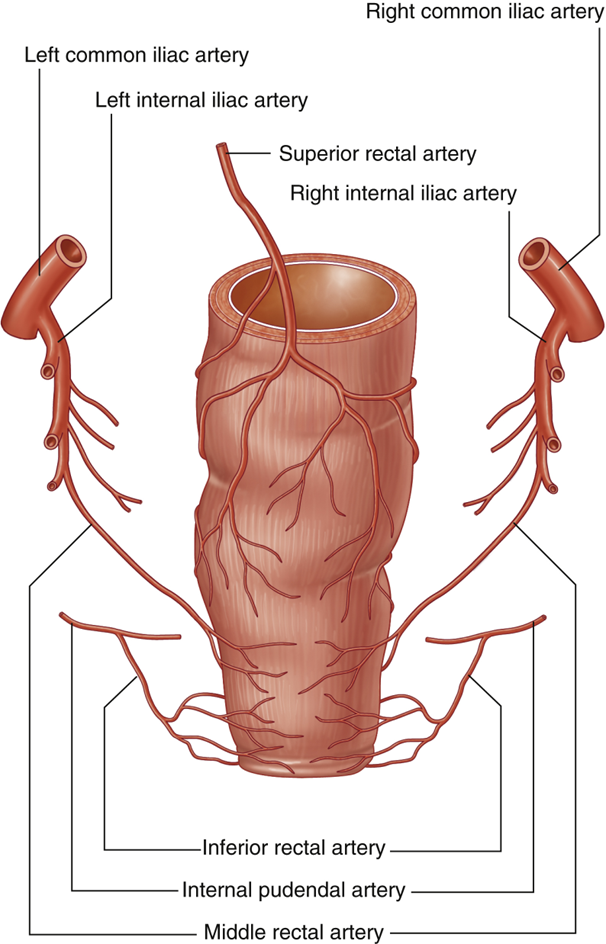
describe the Blood Supply of Rectum & Anal Canal
•Inferior Mesenteric Artery
•Superior Rectal Artery (to pectinate line)
•Internal Iliac Artery
•Middle Rectal Artery
•Forms anastomoses with superior & inferior
•Inferior Rectal Artery
•From internal pudendal
describe the blood draining via the Inferior Mesenteric Vein
•Superior rectal vein
•Drains superior to the pectinate line
•Ascends receiving blood from sigmoid veins and the left colic vein
•These veins accompany arteries of the same name
•Inferior mesenteric vein then joins the splenic vein posterior to the pancreas (can sometimes join the superior mesenteric vein instead)
•Inferior rectal vein:
•Drains inferior to pectinate line
•Drains to internal pudendal vein/ internal iliac (to IVC)

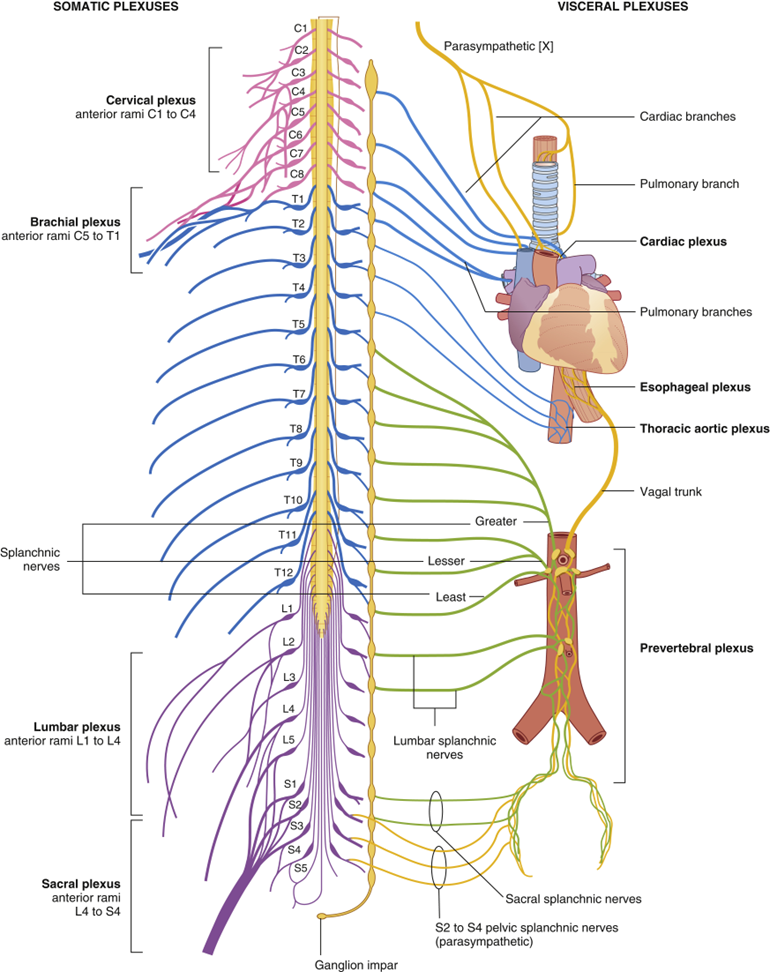
outline the innervation of the colon
•Sympathetic:
•Decrease intestinal activity
•Thoracolumbar Splanchnic nerves
•Jejunum & Ileum – T9 & T10
•Large intestine – T10 – L2
•Parasympathetic:
•Increase intestinal activity
•Craniosacral
•Vagus nerve
•Generally, foregut & midgut
•Pelvic splanchnic nerve (S2- S4)
•Generally, hindgut
explain appendectomy and why is it done
•Inflammation of appendix caused by obstruction (faecal matter or enlarged lymphatic nodules)
•Visceral peritoneum swells resulting in non-specific pain
Removal of the appendix (appendectomy) is usually done laparoscopically
•Incisions in the anterolateral wall for surgical instruments
•Peritoneal cavity inflated with CO2 gas
•Appendix located using the ileocecal junction and point at which 3 teniae converge
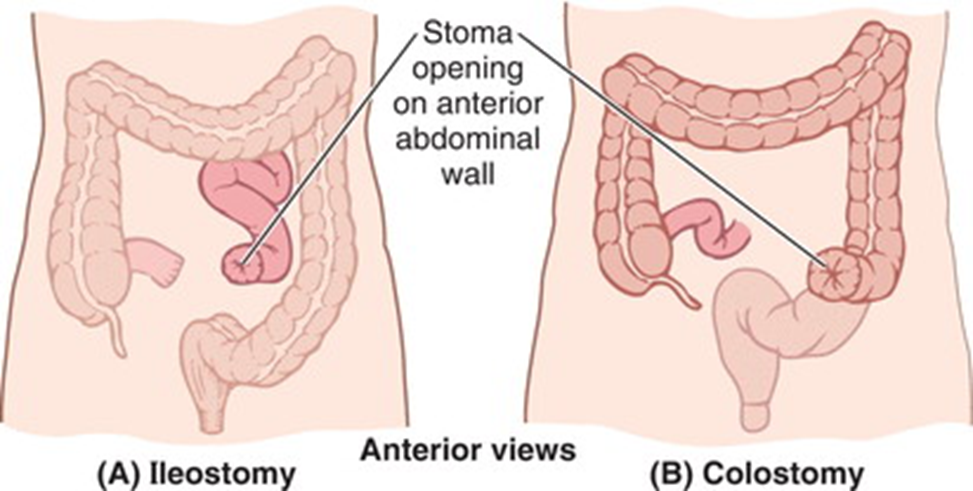
what is a Stoma?
•Ileostomy or colostomy creates a stoma
•Opening of the intestine in the abdominal wall
•Position depends on what part of the intestine is being diverted
•May be permanent or temporary (if allowing intestine to heal)
•A bag is placed over the opening to collect faecal mass
name the sources of GI Infection
uMany GI infections are zoonotic
Symptomatic animals
Economic cost e.g. Salmonella Dublin
Asymptomatic shedders
E.g. reptiles and Salmonella carriage
E.coli O157 in cattle (petting zoos)
uHuman carriers important for some
Typhoid (Mary Mallon)
uEnvironmental sources
Contamination of soil & produce E.g. Listeria, E.coli O157.
understand the different pathogen and their reservoir and environment
Pathogen | Animal reservoir | Food-Borne | Water-Borne |
Escherichia coli | + | + (EHEC) | + (ETEC) |
Salmonella | + | +++ | + |
Campylobacter | + | +++ | + |
Vibrio cholerae | - | + | +++ |
Shigella | - | + | - |
Clostridium perfringens | + | +++ | - |
Bacillus cereus | - | ++ | - |
Vibrio parahaemolyticus | - | ++ | - |
Listeria monocytogenes | ++ | ++ | - |
Yersinia enterocolitica | + | + | - |
explain transmission of GI infection
Faecal-oral
Any means by which infectious organisms from human/animal faeces can gain access to GIT of another susceptible host.
The 3 F’s;
Food
Contamination – farm to fork
Cross-contamination – distribution chain or domestic kitchen
Fluids
Water
Contaminated juices etc.
Fingers
Importance of washing hands;
After toileting
Before and/after preparing or consuming food and drinks
Person-to-person transmission
Infectious dose
Ability to contaminate and persist in the environment > onward transmission.
outline the diagnosis of GI infections
History;
- Nature of diarrhoea, timing (acute Vs chronic), food history (?outbreak), recent antibiotic use, foreign travel.
Examination;
-Febrile, shock, systemically unwell, wasting, neurological signs.
Investigations;
- Blood tests (FBC, UEs, blood film), sigmoidoscopy, abdominal x-ray/CT.
explain the Laboratory diagnosis of GI infection
too many organisms in faeces so is necessary to use various approaches:
Enrichment broth
Contains nutrients that promote preferential growth of the pathogen.
Selective media
Suppress growth of background flora while allowing growth of the pathogen
Differential media
Distinguishes mixed microorganisms on the same plate. Uses biochemical characteristics of microorganisms growing in presence of specific nutrients combined with an indicator that changes colour. Best known examples are Salmonella and Shigella species’ which are non-lactose fermenters (NLF).
describe treatment of GI infections
General points
Most mild bacterial GI infections resolve spontaneously (self-limiting).
Maintenance hydration is crucial & can be life-saving (supportive therapy).
Antibiotic treatment reserved for severe/prolonged symptoms or immunocompromised patients.
Antibiotic therapy itself
May prolong symptom duration.
May exacerbate symptoms.
Promotes emergence of antibiotic resistance.
May actually be harmful e.g. STEC infection.
explain the general control of GI infections
Many organisms have an animal and/or environmental reservoir that cannot be eradicated
Control depends on “breaking the chain” of infection
Adequate public health measures
Provision of safe, clean drinking water
Proper sewage disposal
Education in hygienic food preparation
Hand hygiene
Avoid cross contamination (particularly raw and cooked foods)
Cook foods properly.
Pasteurisation of milk & dairy products.
Sensible travel food practices
Wash it, peel it, cook it or forget it
understand the BIG 3 of GI infections
SALMONELLA, CAMPYLOBACTER, E.COLI
salmonella
Microbiology
Gram-negative bacilli
Member of the Enterobacteriales
Non-lactose fermenters
Epidemiology
Acquired via contaminated food, especially pork, poultry & other meat and milk/dairy products.
Pathogenesis
Diarrhoea due to invasion of epithelial cells in the distal small intestine, and subsequent inflammation
Bacteraemia can occur (extremes of age, immunocompromised).
Distant organs may become seeded to establish metastatic foci of infection e.g. osteomyelitis, septic arthritis, meningitis etc.
Clinical
Incubation 12-72 hours
Watery diarrhoea
Vomiting is common
Fever can occur, and is usually associated with more invasive disease
Duration 2-7 days
Treatment
Fluid replacement is sufficient in most cases
Antibiotics reserved for severe infections and bacteraemia (Typhoid)
Beta-lactams, quinolones or aminoglycosides may be used
Antibiotics and antimotility agents prolong excretion of Salmonellae in the faeces
Specific control points
The introduction of immunisation of poultry flocks
campylobacter
Microbiology
Curved Gram-negative bacilli (gull-wing)
Microaerophilic and thermophilic (42oC)
Epidemiology
Large animal reservoir (poultry, cattle, sheep, rodents & wild birds)
Infection transmitted via contaminated food (esp. poultry), milk or water
Pathogenesis
Inflammation, ulceration & bleeding in small and large bowel due to bacterial invasion
Bacteraemia can occur (extremes of age, immunocompromised)
Rarely causes post-infectious demyelination syndrome (Guillain-Barre), characterised by ascending paralysis
Clinical
Incubation 2-5 days
Bloody diarrhoea
Cramping abdominal pain
Vomiting is not usually a feature
Fever
Duration 2-10 days
Treatment
Fluid replacement is sufficient in most cases
Clarithromycin/erythromycin for severe/persistent disease.
Quinolone (e.g. ciprofloxacin) or aminoglycoside (e.g. gentamicin) for invasive disease
Specific control points
Reduction of contamination in raw, retail poultry meat
Adequate cooking
escherichia coli (E.Coli)
Gram-negative bacilli
Members of the Enterobacteriales
Important component of gut flora of man and animals
Some strains possess virulence factors which enable them to cause infections
Six different diarrhoeagenic groups of E.coli
EPEC (Enteropathogenic E. coli)
Microbiology
No differential media available
Test selection of colonies using polyvalent antisera for common EPEC “O” types
Not routinely done
Epidemiology
Sporadic cases & outbreaks of diarrhoea in infants & children
Cause of some cases of “traveller’s” diarrhoea
ETEC (Enterotoxigenic E. coli)
Microbiology
No differential media available
Test liquid cultures for production of toxins by immunoassays
Not routinely done
Epidemiology
The major bacterial cause of diarrhoea in infants & children in developing world
The major cause of “travellers” diarrhoea
EHEC (Enterohaemorrhagic E. coli)
Microbiology
More than 100 serotypes
Best known is E.coli O157:H7
O157 is a non-sorbitol fermenter. Sorbitol MacConkey agar (SMAC)
Epidemiology
Outbreaks & sporadic cases worldwide (~250 cases/year in Scotland)
Large animal reservoirs (esp. cattle & sheep)
Persistent in environment
Consumption of contaminated food, water and dairy products & direct environmental contact with animal faeces e.g.
petting zoos
Secondary person-to-person spread important (associated with low infectious dose)
TREATMENT OVERALL
Adequate rehydration
Antibiotics not indicated, and in the case of EHEC may increase risk of HUS
Antimotility agents also increase HUS risk
define viral gastroenteritis and at risk people
•inflammation of the stomach and intestines cased by virus(es) and characterised by diarrhoea and vomiting.
•People at higher risk:
–Children under age 5
–Old age people especially in nursing home
–Immunocompromised
name the important viruses that cause gastroenteritis
Norovirus (Norwalk virus)
Sapovirus
• both can affect all ages and healthy individuals but often most serious in young and elderly
Rotavirus
Adenovirus 40 & 41
Astrovirus
• these 3 affect mainly children under 2 years, elderly and immunocompromised
outline norovirus structural features, transmission and clinical features
•Non-enveloped, single stranded RNA virus
Transmission through a variety of routes
–Person to Person (faecal-oral, aerosolised e.g. by toilet flush, fomites)
–Food-borne
–Water
•Infectious dose very small (10-100 virions)
•Can affect all ages
•Very stable and may remain viable for long periods of time in the environment
•24-48 hour incubation period
•Can shed virus for up to 3 weeks after infection
clinical features:
•Can be asymptomatic
•Vomiting (Winter vomiting bug)
•Diarrhoea- non-bloody
•Nausea
•Abdominal cramps
•Headache, muscle aches
•Fever (minority)- usually low grade
•Dehydration in young and elderly
•Usually lasts 12-60 hours
treatment:
•Symptomatic therapy
–Oral &/or IV fluids
–Antispasmodics
–Analgesics
–Antipyretics
outline rotavirus structural features, transmission and clinical features
•Double stranded, non-enveloped RNA virus
•11 strands of RNA so potential for much antigenic variation
transmission:
•Low infectious dose (10–100 virus particles)
•Mainly person to person via faeco-oral or fomites
•Viral shedding of infectious particles can occur in the stool for up to 10 days but can be longer in immunocompromised
•Food and water borne spread is possible
•Spread via respiratory droplets is speculated
clinical features:
•Incubation period 1-3 days
•Clinical manifestations depend on if it is the 1st infection or reinfection
•Symptoms of rotavirus:
•Watery diarrhoea
•Abdominal pain
•Vomiting
•Loss of electrolytes leading to dehydration
•Symptoms usually last 3-7 days
•1st infection after age 3 months is usually the most severe
•Hospital outbreaks in paediatric wards common
complications:
•Severe chronic diarrhoea
•Dehydration
•Electrolyte imbalance
•Metabolic acidosis
•Immunodeficient children may have more severe or persistent disease
Other gastrointestinal complications include: necrotizing enterocolitis
–Common in winter
–Vaccine available
outline adenovirus
•Double stranded DNA virus
•Adenovirus 40 & 41 cause gastroenteritis
•Symptoms: fever and watery diarrhoea
•Treatment: supportive
•No vaccine for adenovirus 40 & 41
•Adeno 40 & 41 and astrovirus behave like rotavirus but rarer
outline astrovirus
•Single stranded, non enveloped RNA virus
•Causes less severe gastroenteritis than other enteric pathogens
•Infection usually as sporadic cases but can be outbreaks, usually in young children
what is Elastase (test) and what do low levels signify?
elastase digests connective tissue. Low stool elastase is a marker of reduced pancreatic enzyme secretion, commonly seen in pancreatic exocrine insufficiency.
what is Hirschprung’s disease?
Failure of neural crest cells to migrate to the colon to form the myenteric and submucosal plexus. this results in a lack of nerve bodies.

what description best explains the diarrhoea seen in non-typhoidal salmonella infection?
Invasion of epithelial cells in the distal small intestine and inflammation
What is a primary function of chloride in the body?
helps regulate acid-base balance
what is the approximate volume of Electrolytes in the intestines (mL/day)?
6,500-8,000 mL/day
Ingestion of which factor could be a cause of osmotic diarrhoea?
lactose
describe the different types of diarrhoea
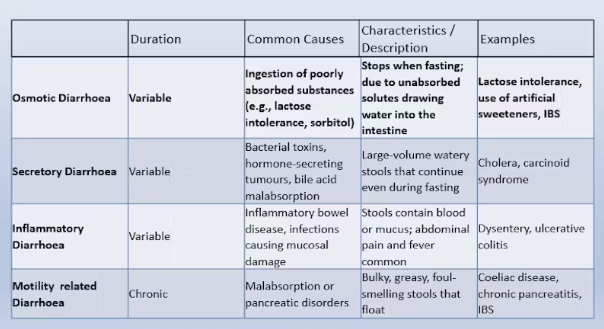
Patients with coeliac disease have a higher risk of cancer. Risk of which cancer is greater in those with poorly managed disease?
non-hodgkin’s lymphoma
what is the gold standard treatment for GI cancers?
surgery.
removing the primary cancer at TNM stage I-III is very effective when combined with chemotherapy or radiotherapy
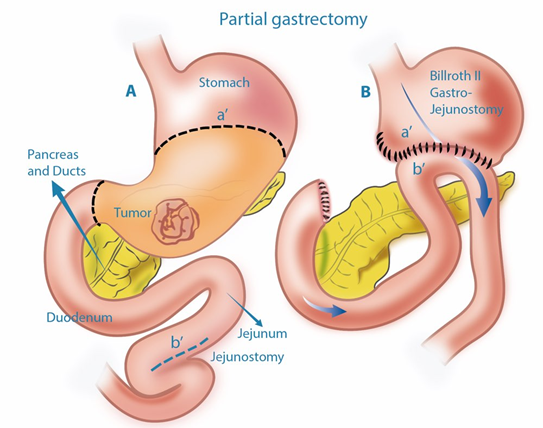
Gastrectomy
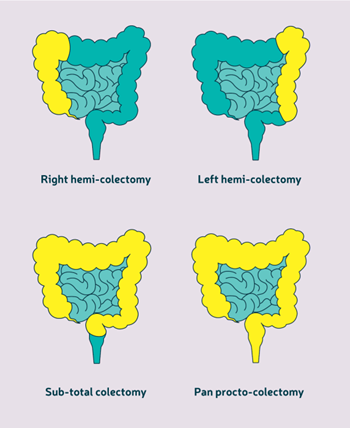
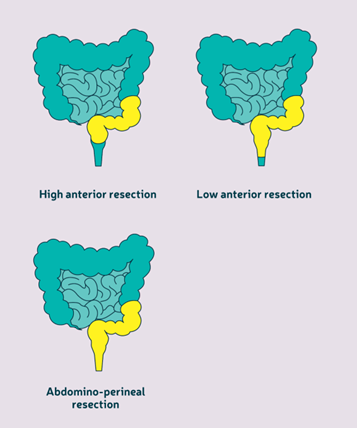
colectomy
describe chemotherapy
•Chemotherapy is a systemic (whole body) therapy given to kill cancer cells themselves by stopping them growing and dividing by causing DNA damage to target the cell for apoptosis through the DNA damage response.
•Chemotherapy targets the cell cycle, when the cell is dividing, so target faster growing cells such as cancer cells, rather than generally slower growing normal cells. However, it cannot distinguish between cancer cells and normal cells that divide quickly so has many side effects such as hair loss.
•Chemotherapy is given in cycles either by intravenous drip or tablets over 3-6 months.
Chemotherapy can be given as an an adjuvant treatment after surgery to remove any remaining cancer cells; as a neoadjuvant treatment before surgery to shrink the tumour to make it easier to remove; or if the cancer has spread it may be given as palliative treatment to ease symptoms
outline common chemotherapy drugs for bowel cancer
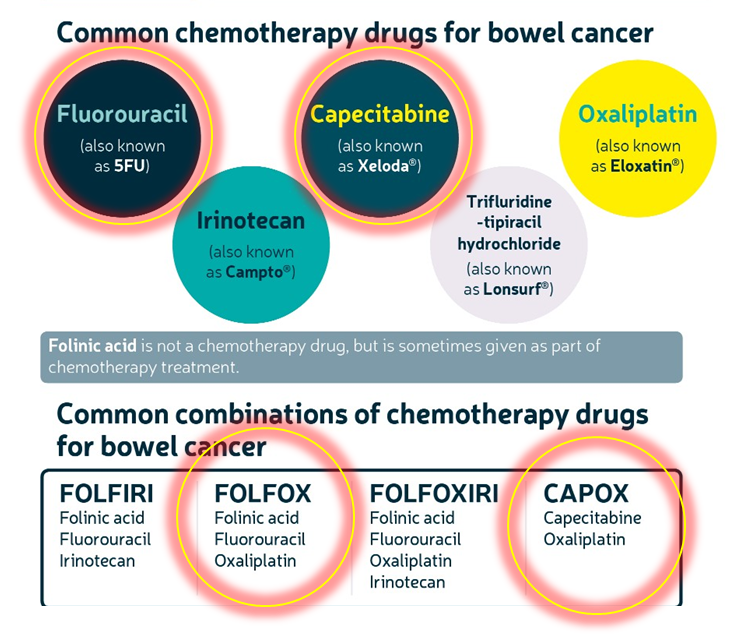
FLUOROURACIL used a lot
CAPECITABINE used often too
describe radiotherapy
•Radiotherapy is when a beam of ionising radiation is focussed on the area of body containing the cancer to induce DNA damage (specifically double strand breaks) to kill the cancer cells directly.
•Similar to chemotherapy it will target the rapidly dividing cells, but it is a localised rather than systemic treatment.
•Radiotherapy is mainly used for rectal cancers within the GI tract; it can be given as either a short course (1-2 weeks) or long course (5-8 weeks) regimen
•Similar to chemotherapy, radiotherapy can be given as an an adjuvant treatment after surgery to remove any remaining cancer cells; as a neoadjuvant treatment before surgery to shrink the tumour to make it easier to remove; or if the cancer has spread it may be given as palliative treatment to ease symptoms.
Radiotherapy can be combined with chemotherapy, known as chemoradiation, to more effectively kill the cancer cells but this regimen has greater short term side effects
describe Targeted therapy in GI cancer
•Targeted therapies are drugs which target a specific aberrant signalling pathway within a cancer cell or the TME (tumour microenvironment)
•This may include drugs that target VEGF, TNFalpha, immune checkpoints or p53
•One common type of targeted therapy is a Receptor Tyrosine Kinase inhibitor
•VEGFR tyrosine kinase inhibitors in hepatocellular cancer
•Immunotherapy in patients with colorectal cancer who have defective mismatch repair
•Antibodies targeting epidermal growth-factor receptors in colorectal cancer patients with K-ras mutations
•Trastuzumab for gastric cancer cancer patients overexpressing HER2
how does Imatinib work?
•a drug which inhibits Abl
Bcr-Abl kinase is working too well usually in CML (chronic myeloid leukemia) but also in high stromal GI cancers
describe precision medicine in GI cancer
•Precision Medicine is the concept of giving the correct treatment to each patient based on individual patient or tumour characteristics
•This can be done at a higher level where patients are placed in groups that receive different treatments – Stratified Medicine
•Or an individual level based on the patient’s specific patient characteristics or genomic make up of the tumour
We don’t have a targeted drug for most patients (yet) – stratified medicine more achievable
outline Immunoediting
Immunoediting is where the tumour cells adapt to have low antigenicity to move from elimination to escape
outline the types of immunotherapy
Immunotherapy aims to enhance the patients own immune system to attack the tumour cells
Immunotherapy can be classified and active or passive

describe examples of active immunotherapy
Checkpoint inhibitors- The most successful immunotherapy. eg. ipilimumab and nivolumab. •These drugs target immune checkpoint proteins CTLA4 and PD-1/PD-L1 (these rely on t-cells being present in the tumour)
PD-L1 is expressed on tumour cells and is the ligand for the PD-1 receptor
PD-L1 acts as a marker for checkpoint inhibition of cytotoxic T-cells
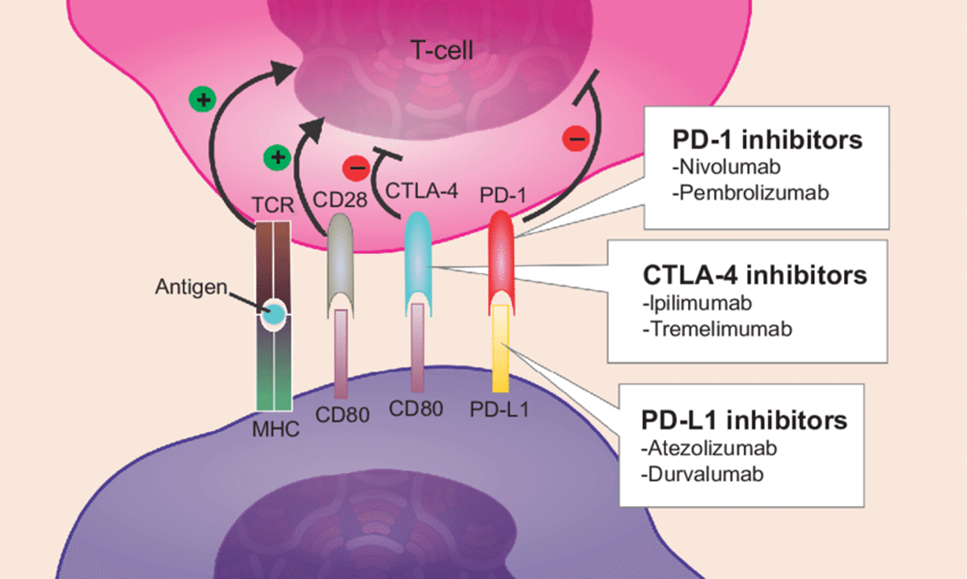
Checkpoint inhibitors for MMR (mismatch repair) deficient GI cancer
drugs: Ipilimumab (CTLA-4 inhibitor), Pembrolizumab (PD-1 inhibitor), Nivolumab (PD-1 inhibitor), Atezolizumab (PD-L1 inhibitor)
describe examples of passive immunotherapy
•The most common passive immunotherapy is adoptive T-cell transfer
•This involves removing a patients T-lymphocyte cells from their blood, adapting them ex vivo and re-infusing them back into the patient
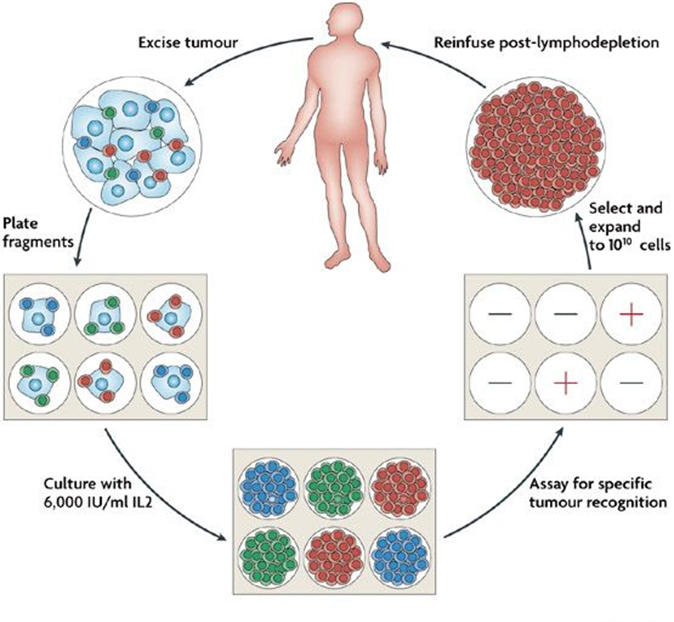
CAR T-cell transfer
Another common passive immunotherapy
•This is the same as adoptive T-cell transfer but utilises a chimeric antigen receptor that does not rely on MHC but is targeted to another cell surface receptor

how do tyrosine kinases work, list common RTKs and drugs that target them in GI cancers?
A tyrosine kinase is an enzyme that transfers a phosphate group from ATP to specific proteins, acting as a cellular "on/off" switch to regulate functions like cell growth, division, and survival.
RTKs are a subclass of tyrosine kinases that are transmembrane receptors, meaning they are located on the cell surface.
nRTK- non receptor-tyrosine kinase
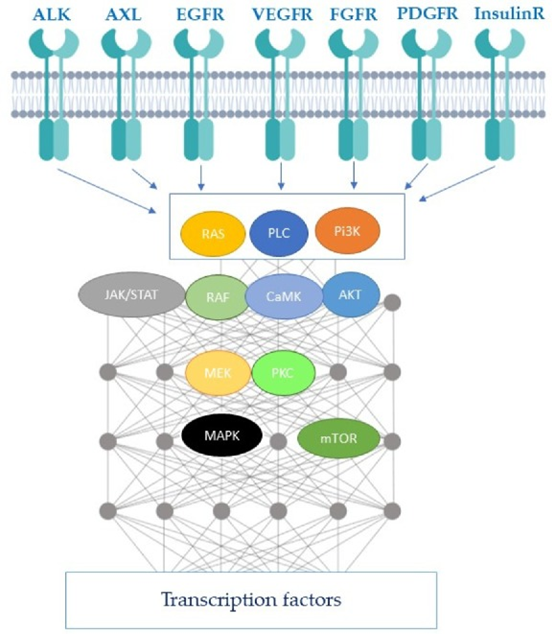
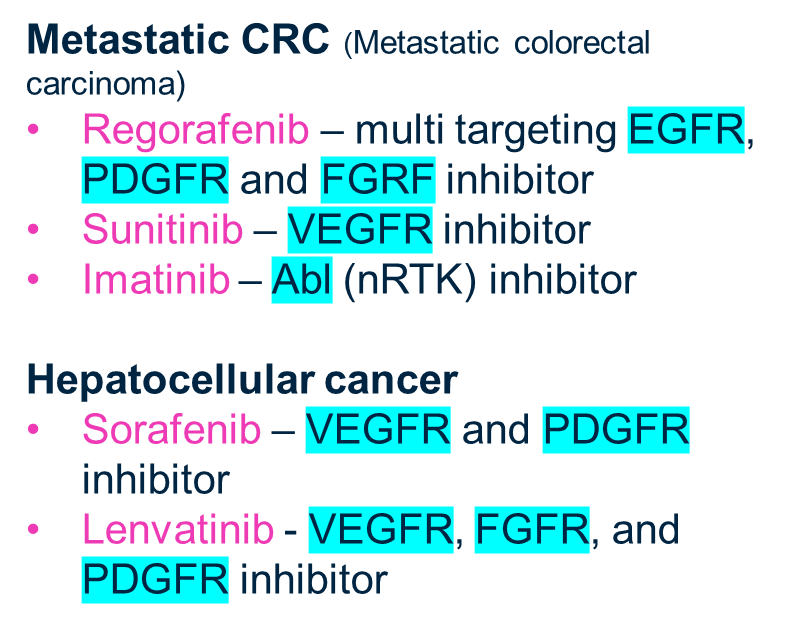
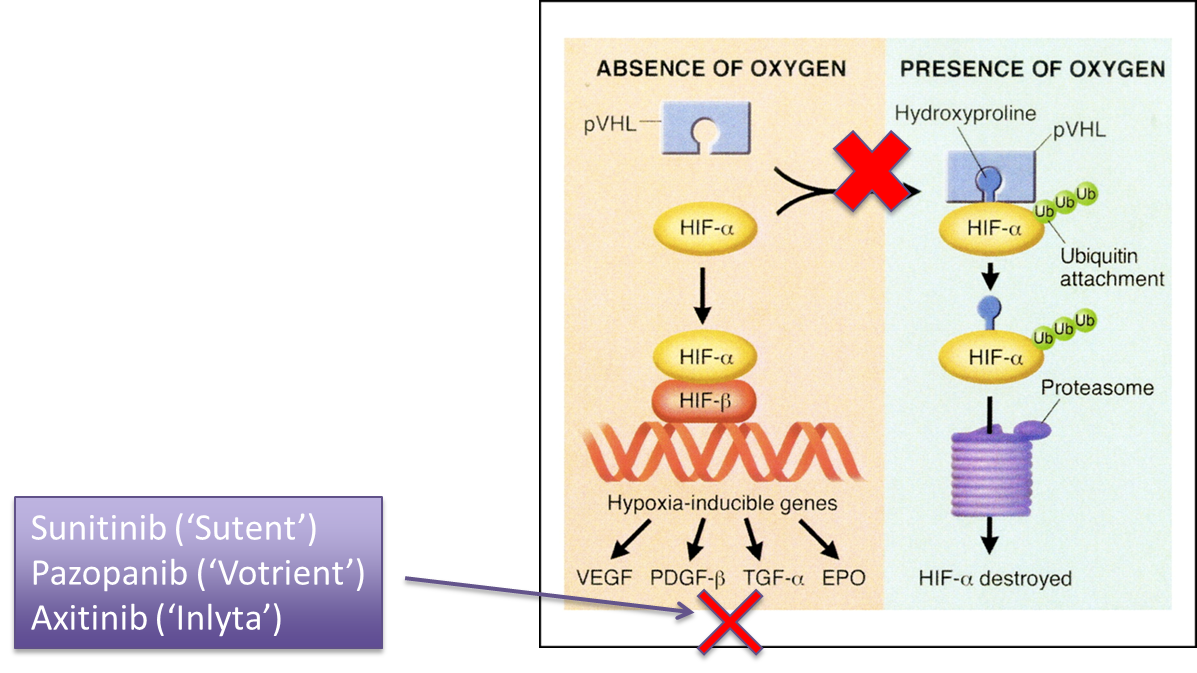
explain Targeting angiogenesis in GI cancer and give drug examples
Tumour’s need oxygen and express factors to induce angiogenesis
If we can inhibit these factors we can cut off the oxygen supply to the tumour
Without oxygen the tumour would undergo necrosis
What are the three key gross anatomical features that distinguish large intestine from small intestine?
Teniae coli (3 longitudinal muscle bands)
haustra (sacculations)
appendices epiploicae (fatty tags).
These adaptations allow for slower transit and water reabsorption.
Which microscopic feature provides a 10-fold increase in surface area in the small intestine?
Villi - finger-like projections covered by enterocytes. The large intestine lacks villi, having a flat surface optimized for water absorption rather than nutrient uptake.
What is the functional significance of Paneth cells found in small intestinal crypts?
Paneth cells produce lysozyme and defensins for antimicrobial defense. Their absence in large intestine contributes to different bacterial flora and susceptibility to certain infections.
How does the goblet cell ratio differ between small and large intestine, and why?
Small intestine has 1:5 goblet cell to enterocyte ratio, while large intestine has 1:1 ratio. The increased goblet cells in LI produce thick mucus to protect against fecal abrasion and facilitate stool passage.
What histological finding would confirm a biopsy is from the small intestine rather than large intestine?
Presence of villi and Paneth cells in the crypt bases.
Large intestine shows no villi, shallow crypts, and abundant goblet cells throughout.
Which specific transporter is responsible for glucose absorption in enterocytes?
SGLT1 (Sodium-Glucose Linked Transporter 1) at the apical membrane. This sodium-dependent mechanism explains why glucose absorption requires concurrent sodium transport.
What is the clinical significance of Brunner's glands in the duodenum?
They secrete alkaline mucus (pH 8.5) that neutralizes gastric acid. Loss of this function contributes to peptic ulcer disease in the first part of duodenum.
Where specifically in the small intestine does vitamin B12 absorption occur?
Terminal ileum via intrinsic factor (IF) receptors on enterocytes. This explains why patients with terminal ileal resection or Crohn's disease develop B12 deficiency.
Stomach: IF binds to B12.
Small Intestine (Ileum): The IF-B12 complex binds to Cubilin (IF receptors) on ileal cells.
Internalization: Cubilin facilitates endocytosis of the complex.
Transport: Inside the cell, B12 is released from IF and transferred to another protein (Transcobalamin II) for transport in the blood.
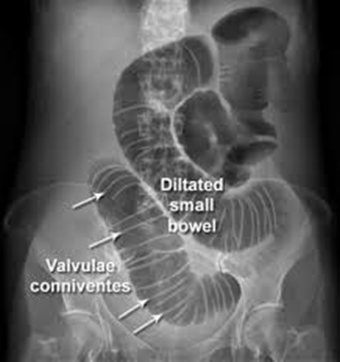
What CT finding distinguishes jejunal loops from other small bowel segments?
Valvulae conniventes - parallel lines visible within the lumen representing circular folds. These are most prominent in jejunum and help identify the segment.
A patient with celiac disease has villous atrophy on biopsy. What specific histological changes would you expect?
Flattened villi (villous atrophy), crypt hyperplasia (deep crypts), increased intraepithelial lymphocytes, and increased mitotic figures (dividing cells) in crypts. These changes reduce absorptive surface area.
define diverticulitis
colonic diverticula- little pouches form in the inside lining of your colon. They usually don't cause any problems. But rarely, they may bleed or develop an infection (diverticulitis).
Does the colon have villi, what’s the Functional implication of this?
No, only glands.
Colon absorbs water and secretes mucus, not nutrients.
What is the main histological feature of the large intestine, and the functional implication?
Long glands with many goblet cells.
Mucus lubricates faeces for smooth passage
How do glands differ between small and large intestine?
Small intestine: shorter, less developed.
Large intestine: longer, well‑developed, filled with goblet cells.
What are the teniae coli and their functional implication?
Three longitudinal muscle bands in the colon.
Cause haustrations seen on imaging
How are muscular layers arranged in the colon?
Inner circular (thick), outer longitudinal (thins except at teniae coli).
Where is lymphoid tissue found in the intestine and the Functional implication?
Common in mucosa/submucosa of colon and abundant in appendix.
provides immune defence against pathogens in lumen.
How does the appendix differ histologically from the colon and the Functional implication?
Same mucosa (no villi, elongated glands), but fewer glands and more lymphoid tissue.
Acts as immune tissue reservoir.
What feature distinguishes the colon vs the small intestine on imaging?
Haustrations caused by teniae coli contractions in the large intestine.
Plicae circulares (folds), villi not visible grossly in small intestine
How is gastric acid chyme neutralised in the duodenum?
Pancreatic secretions rich in bicarbonate (alkaline) neutralise acid → optimise enzyme activity.
Which enzyme digests starch in the small intestine?
Pancreatic α‑amylase→ breaks α‑1,4 bonds in starch → resulting in maltose, maltotriose, dextrins
What causes bile acid diarrhoea?
Ileal disease or resection → bile acids irritate colon.
What condition causes enzyme deficiency and malabsorption of fats/proteins?
Chronic pancreatitis → pancreatic insufficiency → steatorrhoea, protein malabsorption.
Which brush border enzymes complete carbohydrate digestion?
Maltase, isomaltase, sucrase, lactase (on intestinal epithelial cells)
What sugars are digested by brush border enzymes?
Sucrose (by sucrase), lactose (by lactase), maltose (by maltase).
What happens to lactose digestion with age?
Lactase activity declines in many populations → lactose intolerance; persists in ~90% Northern Europeans.
Which carbohydrates cannot be digested by human enzymes?
Cellulose, raffinose, stachyose → fermented by colonic bacteria.
Which enzyme digests fats?
Pancreatic lipase (with bile salts and co-lipase).
where bile salts emulsify fats, but inhibit lipase; colipase then acts as a cofactor, binding lipase and allowing it to attach to fat droplets (triglycerides) in the gut to break them down into monoglycerides and fatty acids for absorption, forming a vital lipolytic complex.
What is the role of bile salts in fat digestion?
Emulsify lipids, coat micelles, enhance lipase activity; undergo enterohepatic circulation.
Where are bile salts reabsorbed?
Terminal ileum;
disease/resection (e.g. Crohn’s) → bile acid diarrhoea.
Which enzymes digest proteins in the stomach?
Gastric pepsin (breaks ~40% of peptide bonds).
Which pancreatic proteases digest proteins in the small intestine?
Trypsin (bonds next to lysine, arginine), chymotrypsin (bonds next to large hydrophobic residues e.g. tryptophan, tyrosine, isoleucine), elastase (bonds next to small hydrophobic residues e.g. alanine).
What controls fasting motility in the small intestine?
Migrating motor complex (MMC) clears residual contents.
What are the two post‑prandial motility patterns in the small intestine?
Segmentation (ring contractions, mixing chyme) and peristalsis (propulsion distally).
What is post‑operative ileus?
Temporary paralysis of motility after abdominal surgery (small intestine ~24h, stomach ~48h, colon ~72h).
What are haustral contractions?
Ring‑like contractions in colon → mixing, water absorption, bacterial fermentation.
What are mass movements in the colon?
Long peristaltic contractions propelling faeces into rectum.
What structures cause haustrations in the colon?
Teniae coli (three longitudinal muscle bands).
What triggers colonic mass movements?
Morning waking and meals (gastrocolic reflex)
Which drugs reduce colonic motility?
Opiates (loperamide, codeine)
Anticholinergics.
Which drugs increase colonic motility?
Stimulant laxatives (senna, bisacodyl).
Prucalopride
Linaclotide
Which anal sphincter is involuntary?
Internal anal sphincter (smooth muscle, tonic contraction).
Which anal sphincter is voluntary?
External anal sphincter (skeletal muscle, pudendal nerve).