Edexcel International A level Chemistry: Unit 2
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
146 Terms
exothermic
a chemical reaction in which heat energy is transferred from the system to the surroundings
endothermic
a chemical reaction in which heat energy is transferred from the surroundings to the system
enthalpy
the sum of the system's internal energy and the product of its pressure and volume. Denoted by H and measured in Joules.
H = U + PV
where U = internal energy. The inclusion of the PV term corrects for the work done on or by the surrounding atmosphere if the system volume changes
Conceptually, to create the system "in place", we'd first have to push back the surrounding atmosphere to make a vacuum big enough for it (which takes PV), then create the system in that empty space (which takes U).
specific heat capacity
the energy required to raise the temperature of one gram of a substance by 1K. (in chemistry the unit of mass is usually the gram).
Used as:
∆Q = mc ∆T
where
∆Q : heat supplied
∆T : temperature change
standard enthalpy change of reaction
The enthalpy change which occurs when equation quantities of materials react under standard conditions
Enthalpy level diagram
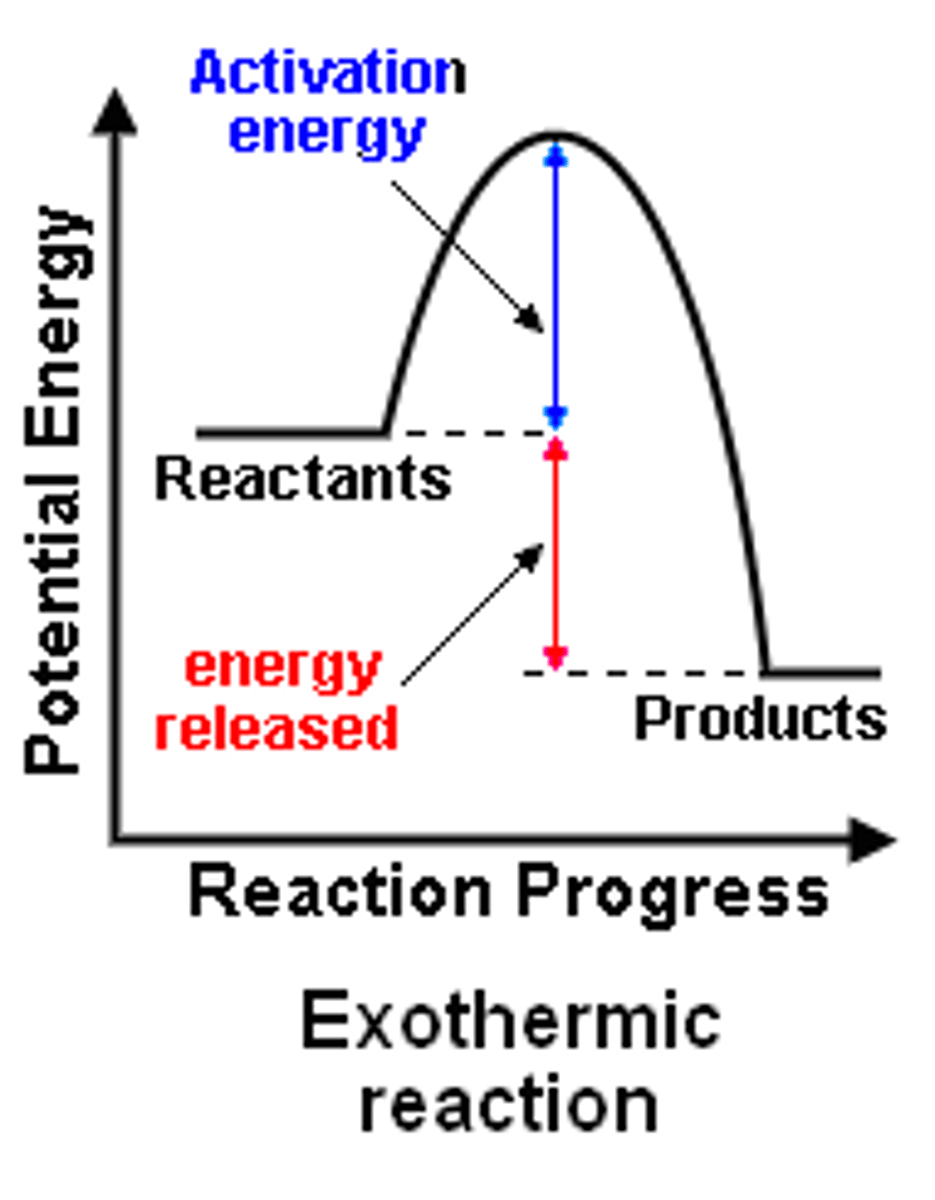
enthalpy change
the amount of energy absorbed or lost by a system as heat during a process at constant pressure
it is NEGATIVE for EXOTHERMIC processes

Thermometric Titration
A titration where the endpoint is indicated by a temperature change
Standard enthalpy change of combustion
The enthalpy change when one mole of a substance is completely burned in oxygen, under standard conditions (100kPa, 298K)
standard enthalpy change of neutralisation
The enthalpy change when an acid and alkali react together to form one mole of water, under standard conditions (100kPa, 298K)
standard enthalpy of formation
the change in enthalpy when one mole of a substance forms from its elements, with all substances in their standard states at standard conditions (100kPa, 298K)
standard states
Physical states under standard conditions
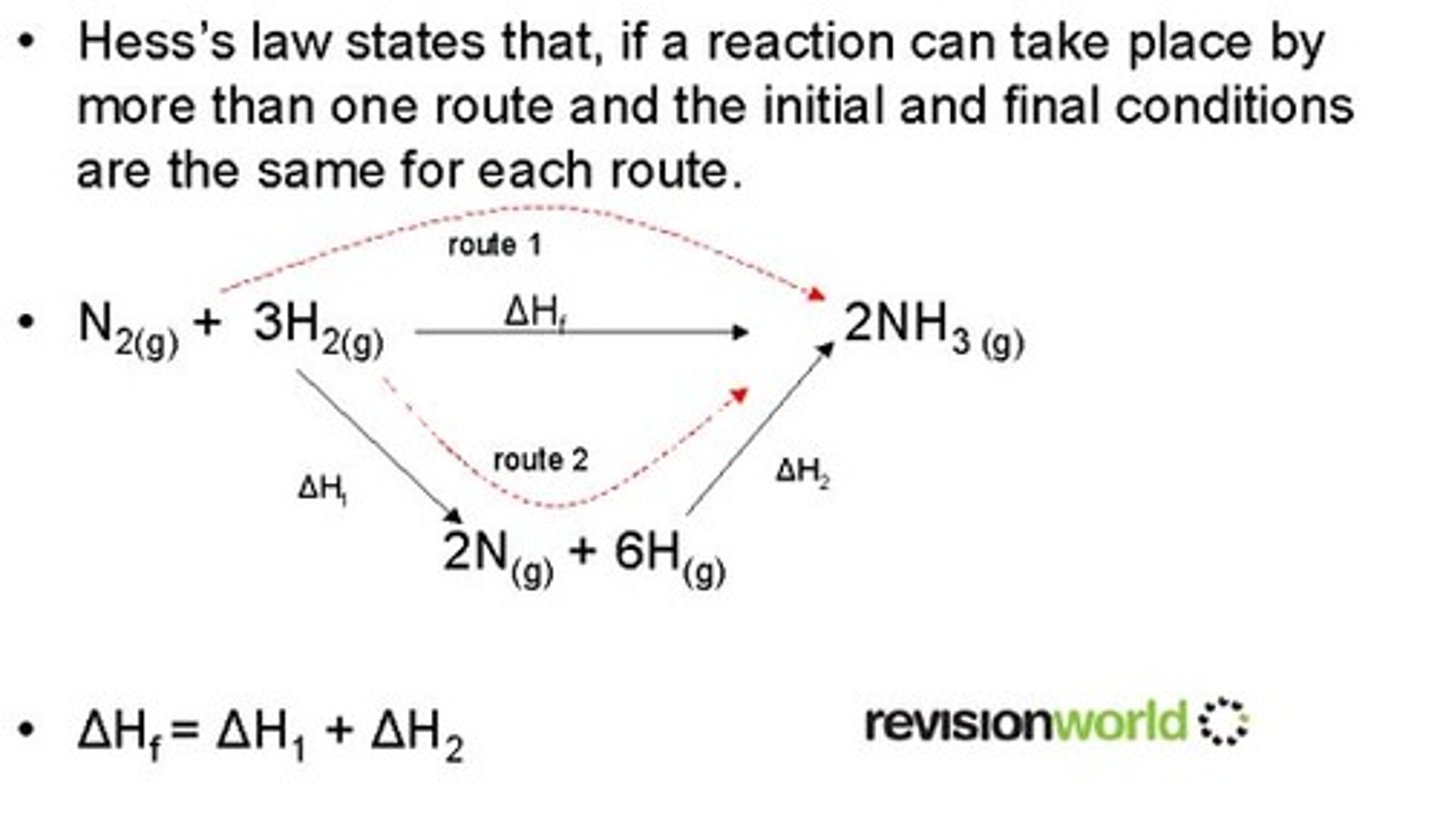
Hess's Law
the overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process
when reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps and is therefore independent of the path
This is a result of conservation of energy
bond enthalpy
The enthalpy change when one mole of a bond is broken in the gaseous state
measured in Joules/mol or kJ/mol
mean bond enthalpy
The enthalpy change when one mole of a bond is broken in the gaseous state, averaged over many molecules.
convenient unit : kJ/mol
standard enthalpy change of atomisation
The enthalpy change that takes place when one mole of gaseous atoms forms from the element in its standard state, measured at stated conditions (usually 298K and 100kPa).
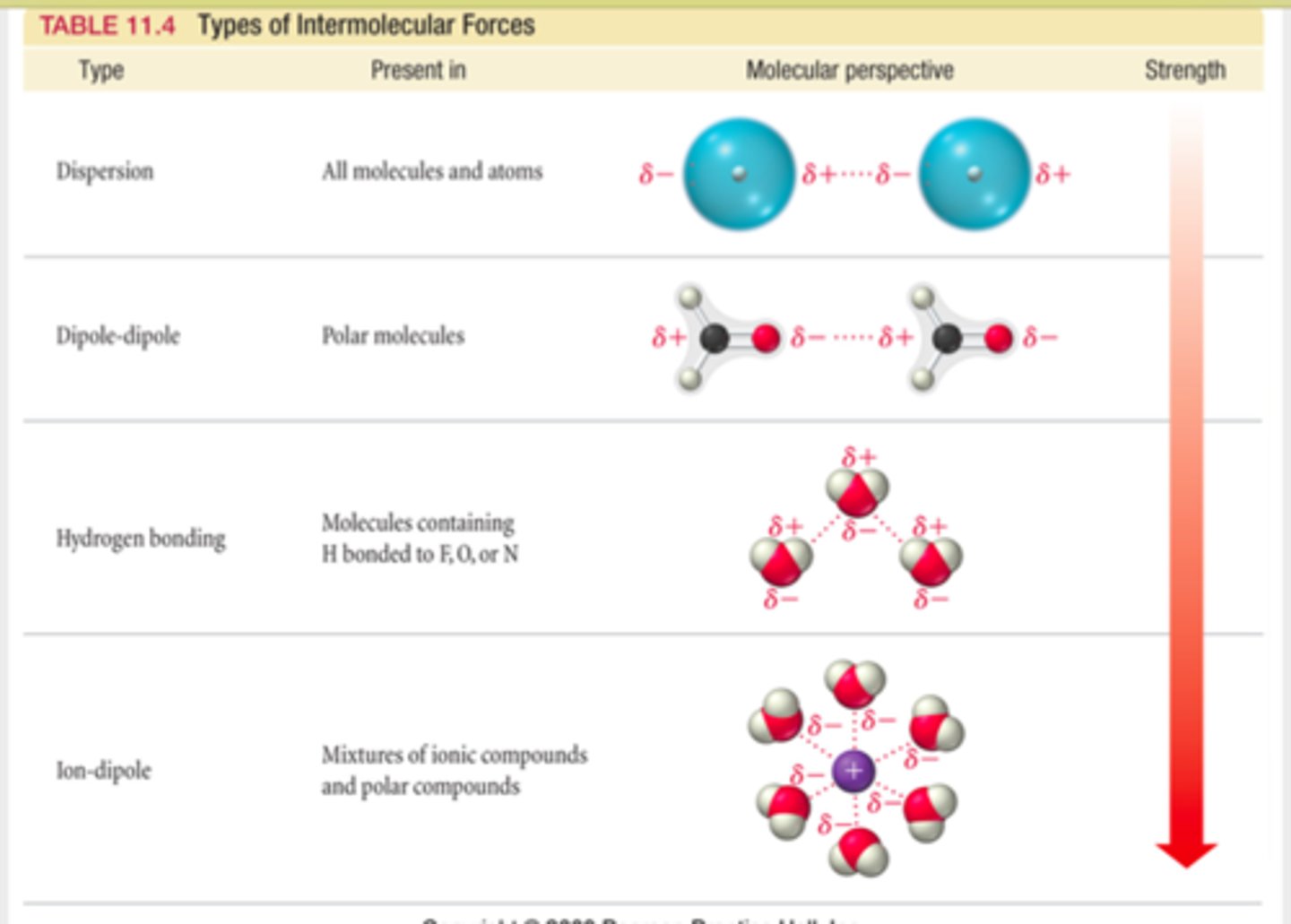
intermolecular forces
forces of attraction between molecules in substances with simple molecular structures
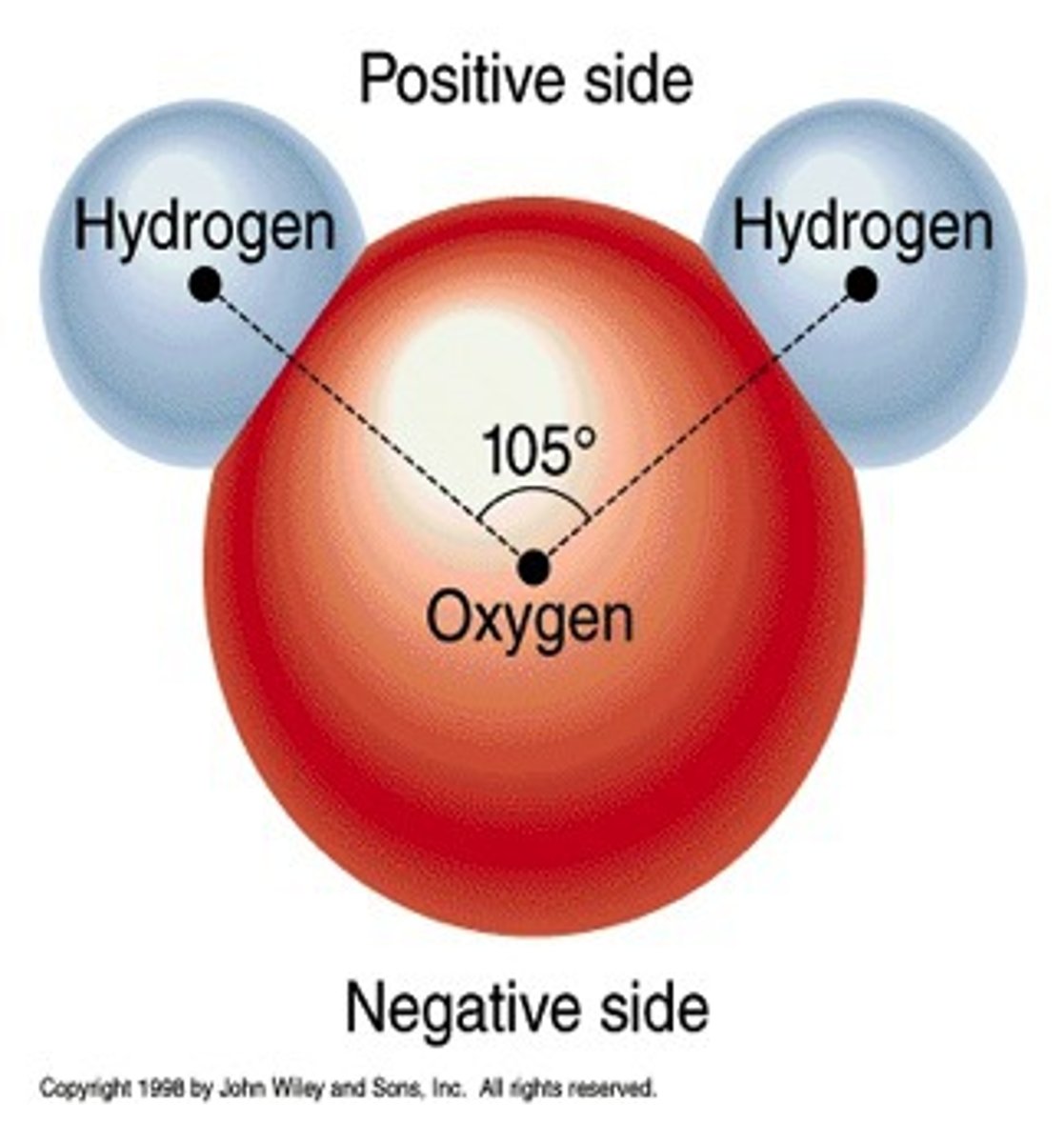
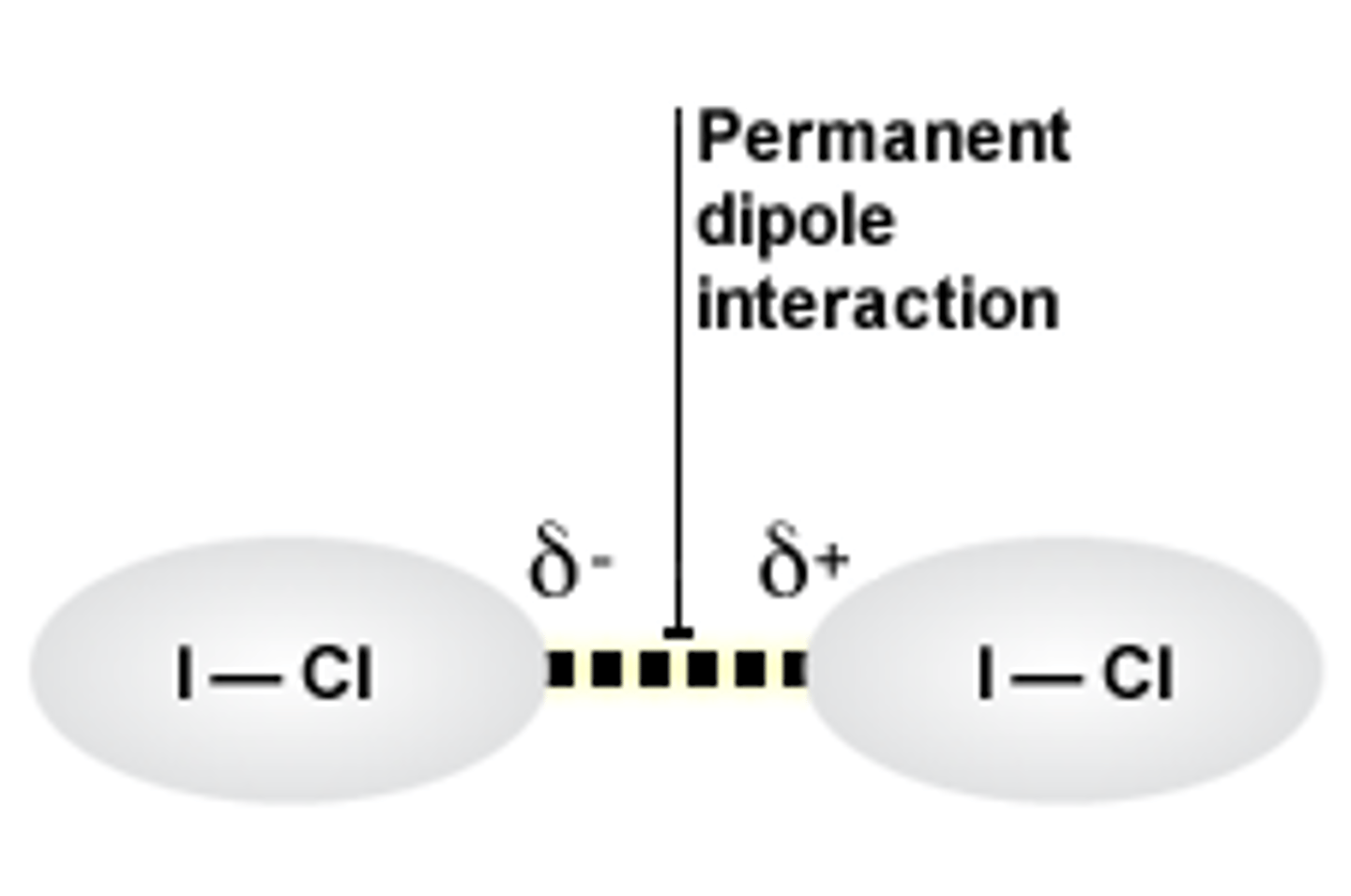
permanent dipole
A small charge difference across a bond resulting from a difference in electronegativities of the bonded atoms.
Electronegativity
a measure of the tendency of an atom to attract a bonding pair of electrons. Differences in this quantity between atoms therefore account for the degree of ionic or polar character of the resulting bond
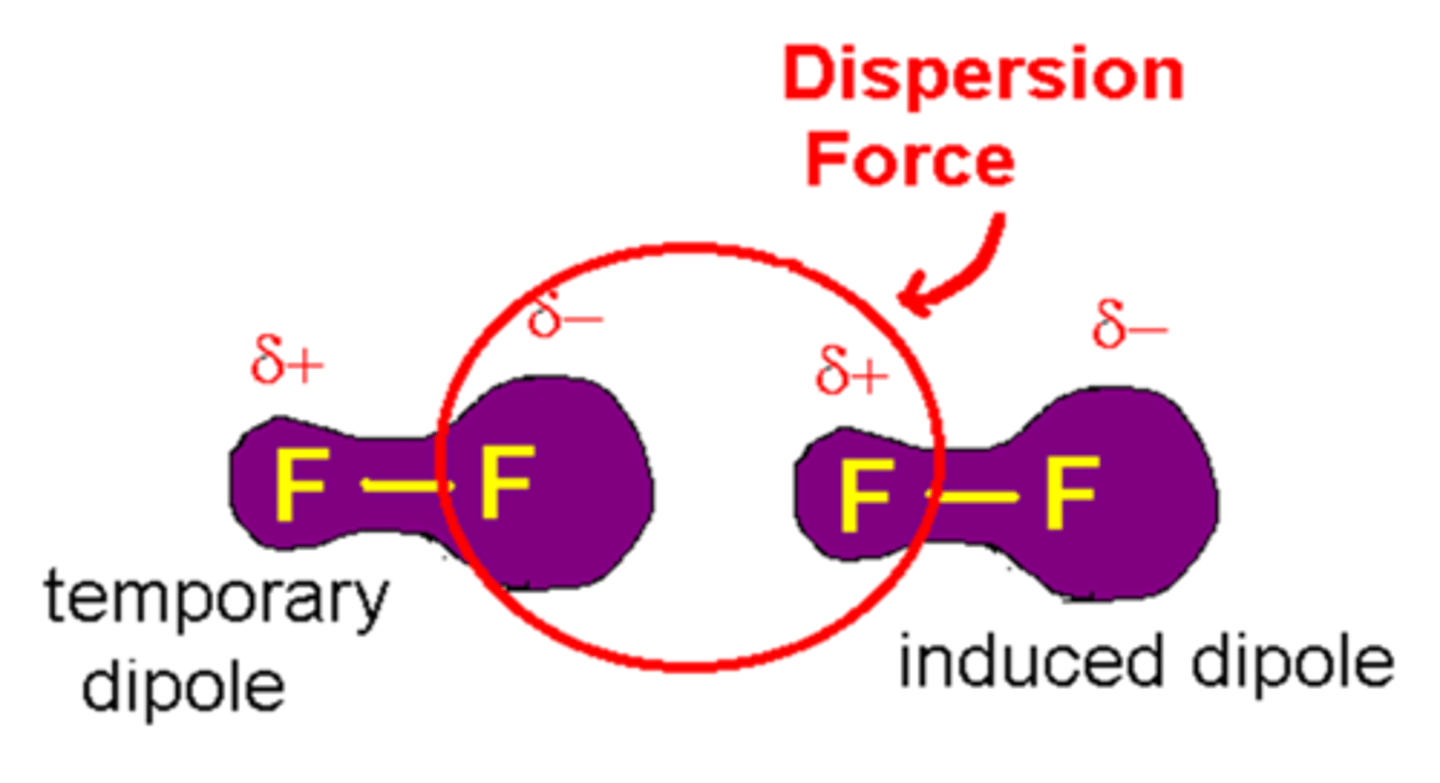
London Dispersion forces
the intermolecular attraction resulting from the uneven distribution of electrons and the creation of temporary dipoles
an instantaneous dipole on one molecule can induce a second dipole on a neighbouring molecule, resulting in an electrostatic attraction, described as an
instantaneous dipole-induced dipole interaction
This interaction is why we can liquefy e.g. noble gases.
Interaction strength increases with the size and polarisability of the electron cloud
Permanent dipole-dipole interactions
An attractive force between permanent dipoles in neighbouring polar molecules.
The strength of London dispersion forces
Depends on the Size and and Shape.
Size:
larger atoms/ molecules are more polarizable than smaller atoms/ molecules (larger e- cloud)
chain length (molecule shape):
longer chains -> ability to have good alignment, more points of close proximity -> stronger attraction
Branching
more branching -> poorer alignment so fewer points of close proximity-> weaker attraction
van der Waals interaction
a term which is generally taken to refer to all intermolecular forces
it's often better to be more specific and say either London Dispersion OR permanent dipole - permanent dipole etc.
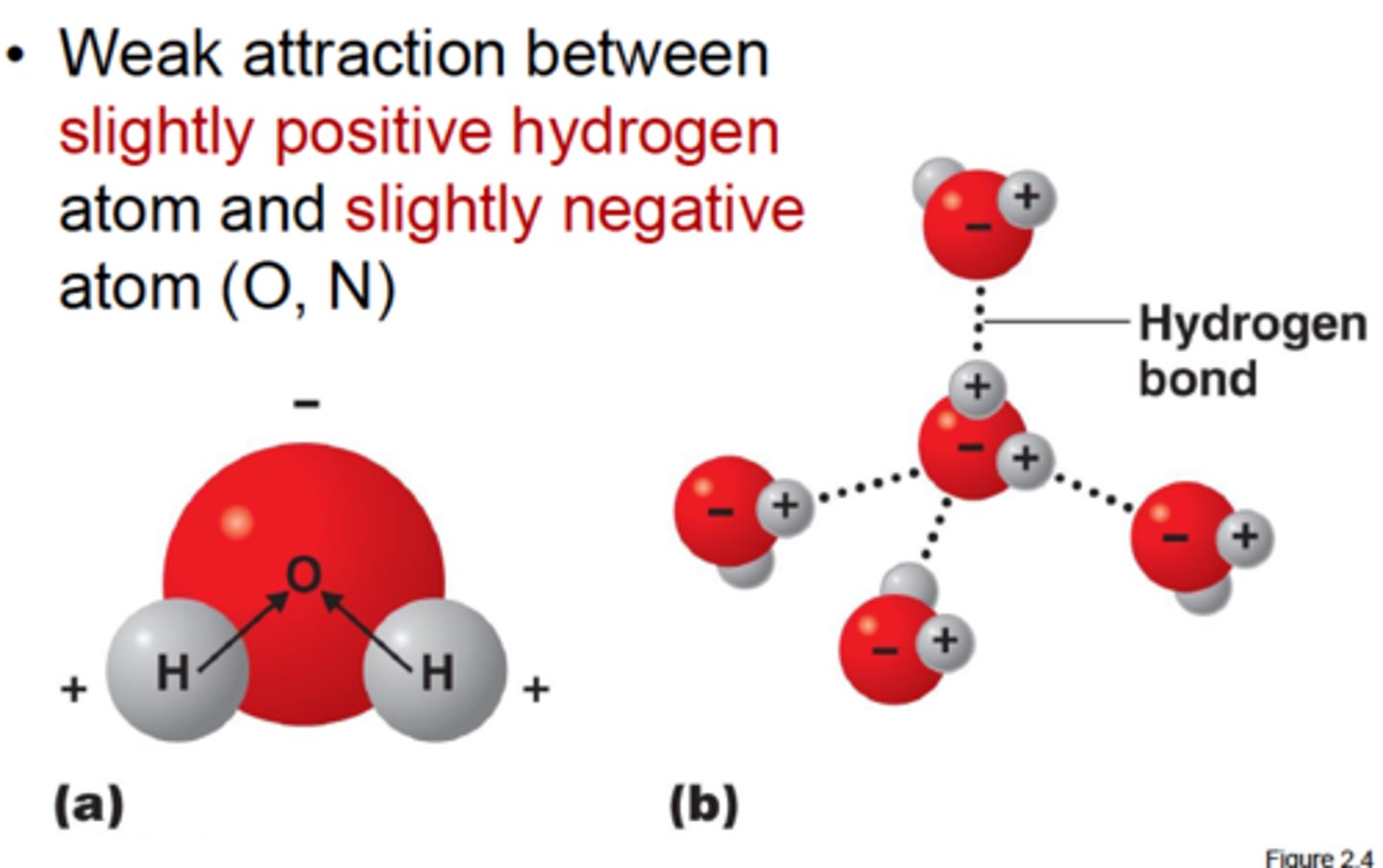
Hydrogen Bond
A type of weak chemical bond formed when the slightly positive hydrogen atom of a polar covalent bond in one molecule is attracted to the slightly negative atom of a polar covalent bond in another molecule.
conditions: to form, we must have:
a) hydrogen bonded to a very electronegative element (F, O, CL), which pulls the electron cloud away from the proton
b) A partially negative atom that is able to approach the exposed proton.
polar solvent
A dissolving liquid composed of polar molecules
nonpolar solvent
a dissolving liquid composed of nonpolar molecules
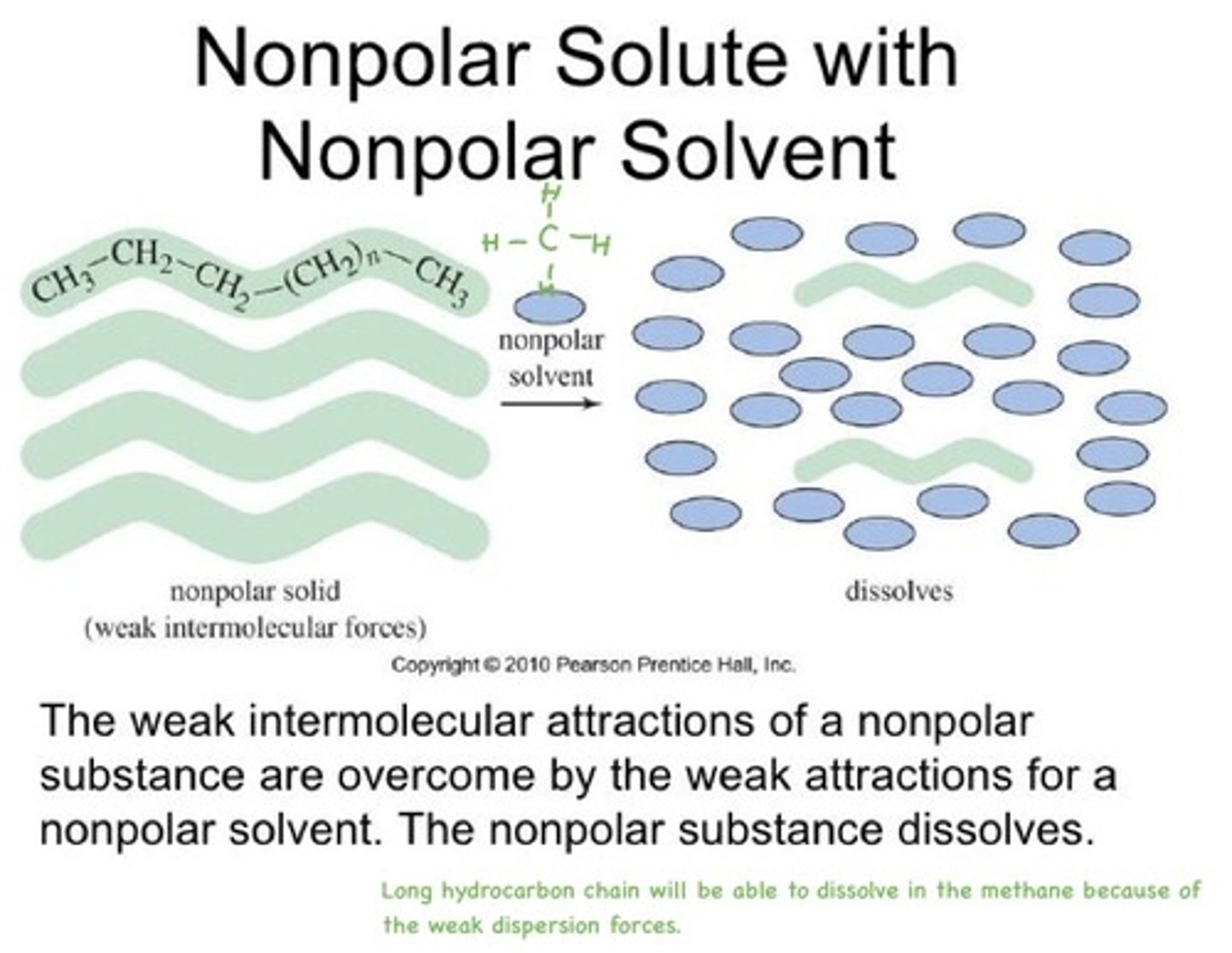
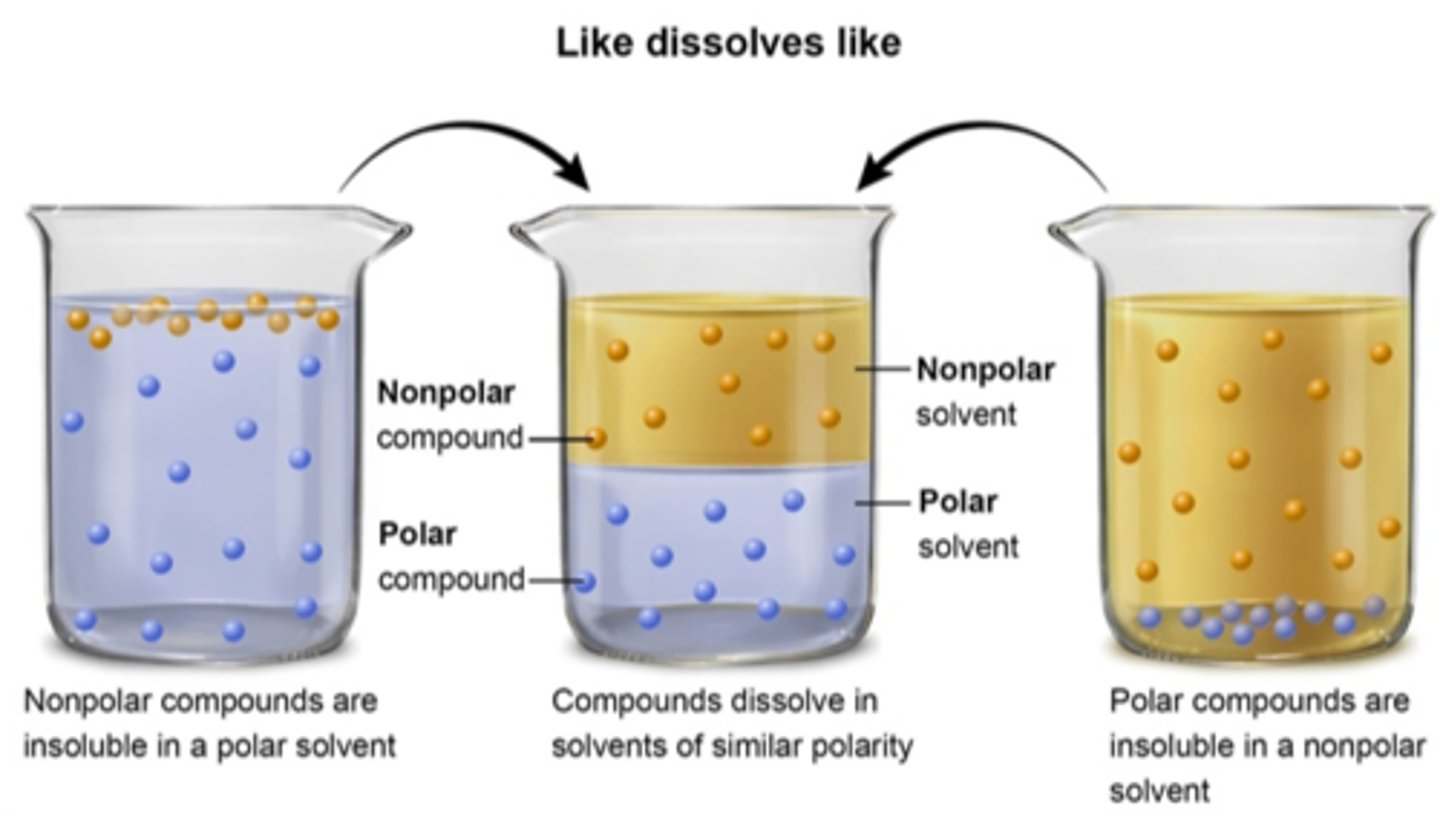
like dissolves like
polar and ionic solutes will dissolve in polar solvents
nonpolar solutes will dissolve in nonpolar solvents
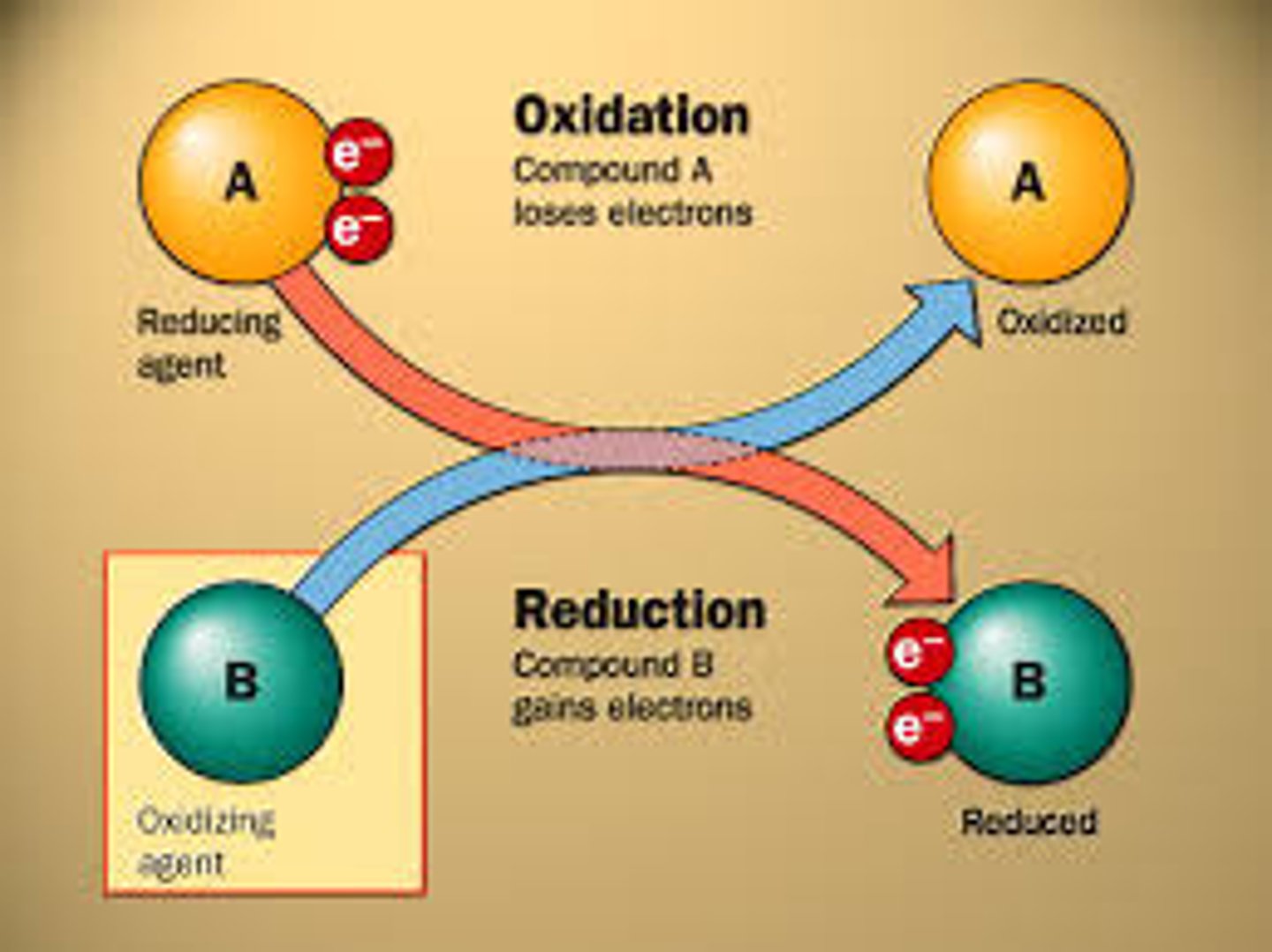
reduction
Gain of electrons
(old definition : the removal of oxygen or addition of hydrogen)
the oxidation number decreases
123HFOCl
Oxidation Number mnemonic
remember:
use roman numerals for oxidation numbers
Cl stands for chlorine, not carbon
oxidation number rules

oxidation
loss of electrons
(old definition: the addition of oxygen or removal of hydrogen)
the oxidation number increases
redox reaction
a chemical reaction that involves both reduction and oxidation
oxidising agent
A reagent that oxidises (takes electrons from) another species (and is itself reduced)
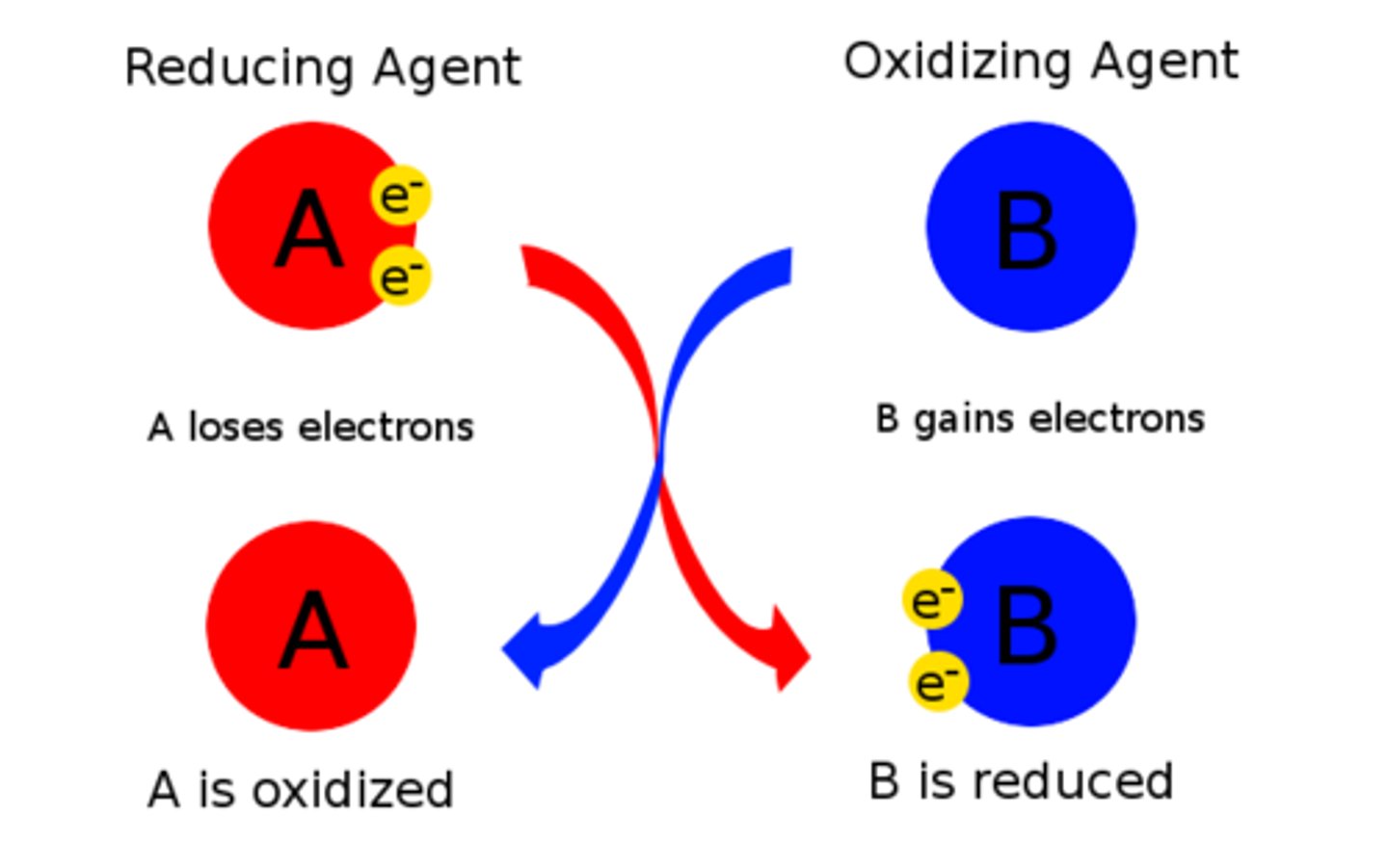
reducing agent
Reagent that donates electrons to another species and becomes oxidized.
Disproportionation
A redox reaction in which the same element is simultaneously oxidised and reduced leading the the oxidation numbers to become (more) unequal
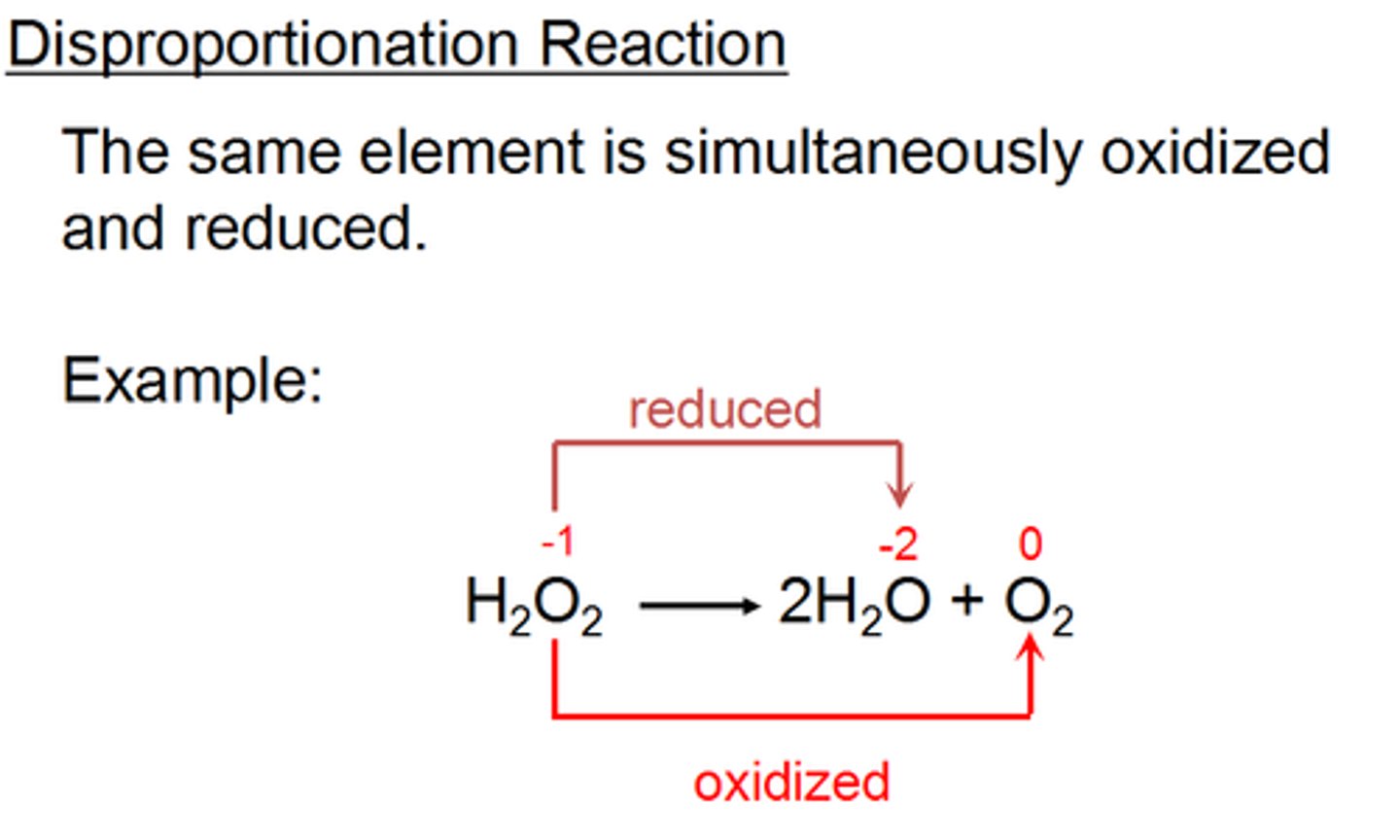
Comproportionation
the reverse of disproportionation, two species of the same element in different oxidation states will form a common product of the same oxidation state (or the difference in their oxidation states becomes smaller).
First ionisation energy
The energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions
Second ionisation energy
The energy required to remove one electron from each ion in one mole of gaseous singly charged 1+ ions to form one mole of gaseous 2+ ions.
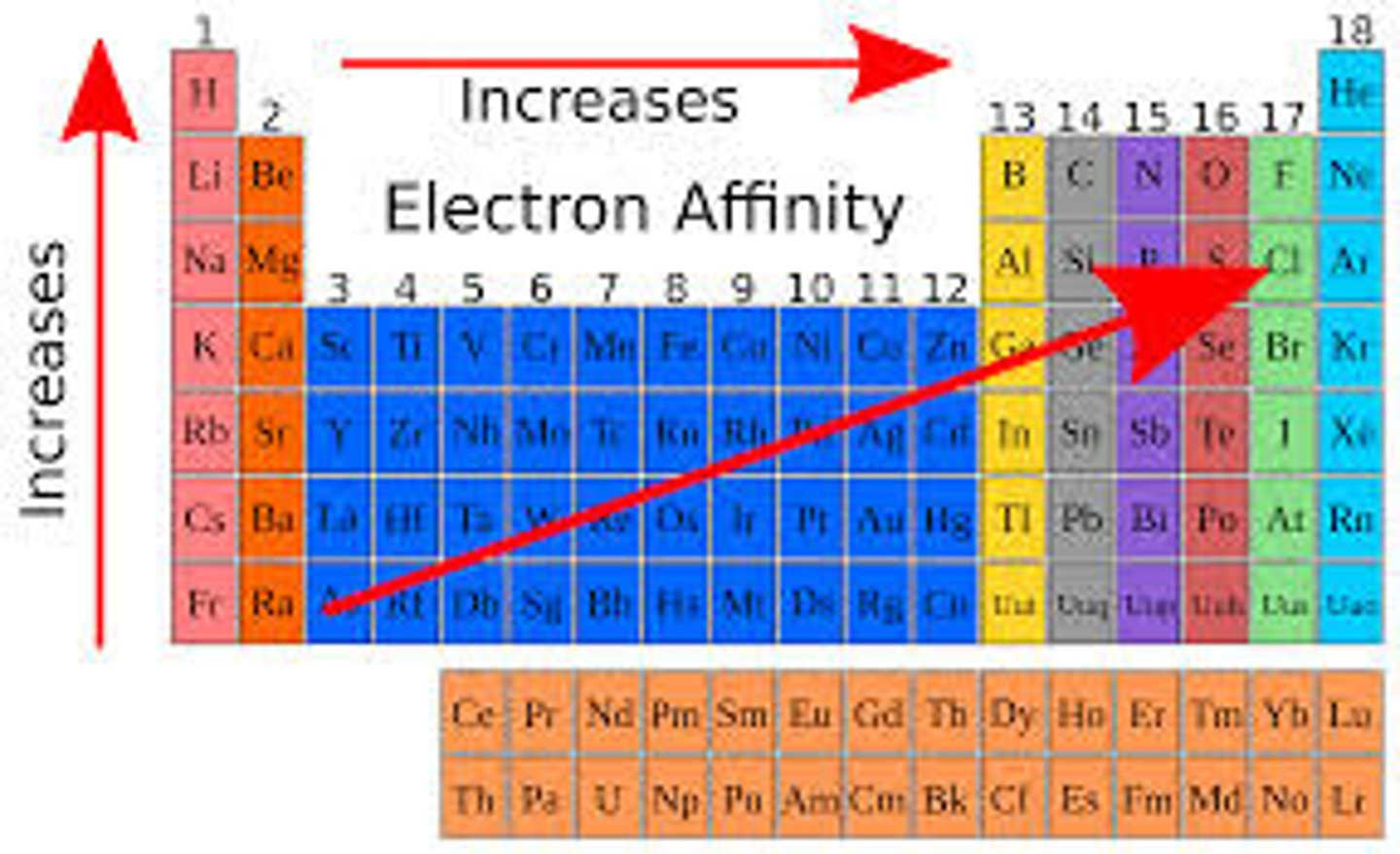
First electron affinity
The enthalpy change that takes place when one electron is added to each atom in one mole of gaseous atoms to form one mole of gaseous 1- ions
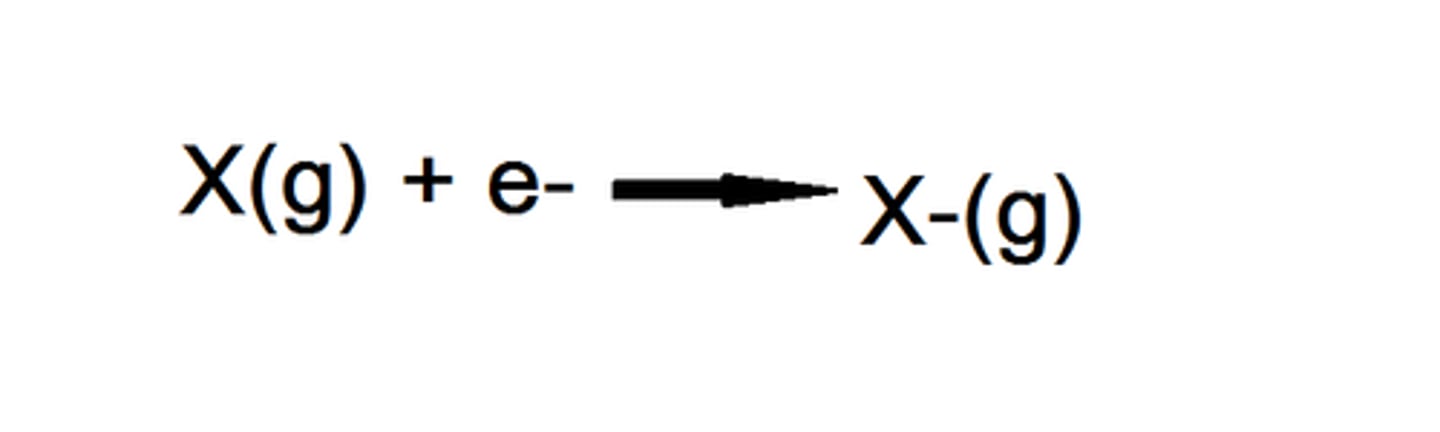
Second electron affinity
The enthalpy change that takes place when one electron is added to each ion in one mole of gaseous 1- ions to form one mole of gaseous 2- ions.
Lattice enthalpy
The enthalpy change that accompanies the formation of one mole of an ionic compound from its separated gaseous ions under standard conditions
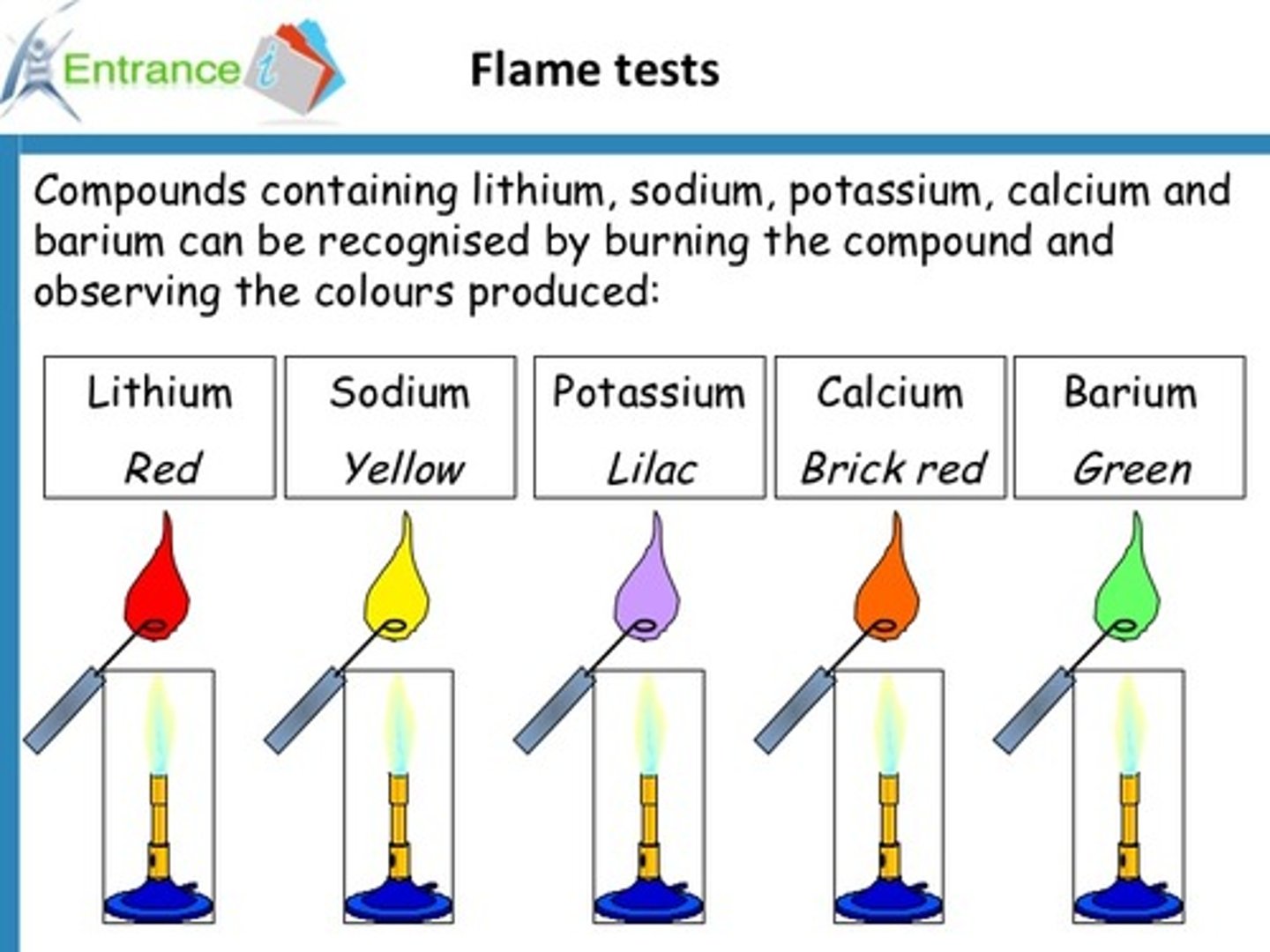
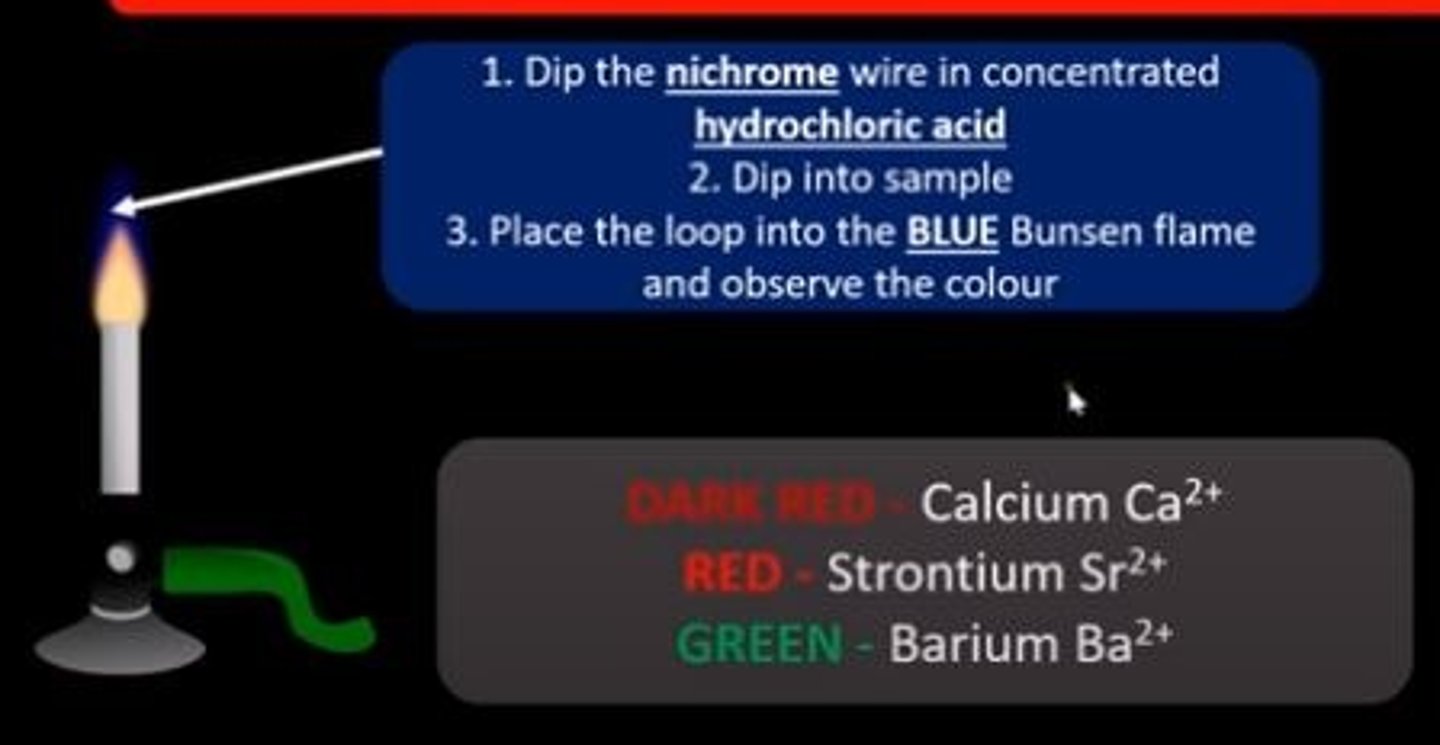
flame tests
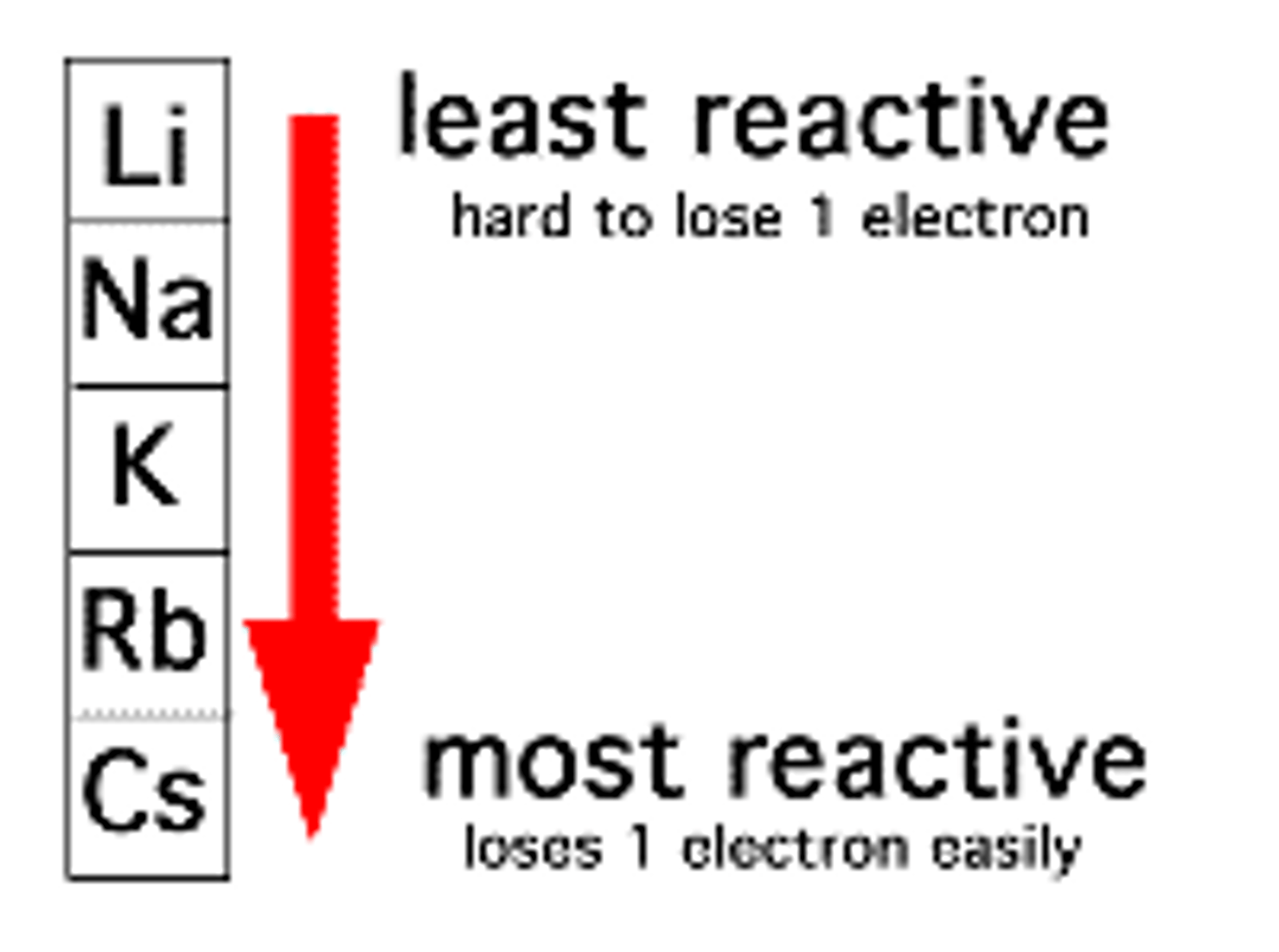
Group 1 reactivity
- Down the group, atomic radii increases.
- Therefore, there is less nuclear density.
- Attraction from the positive nucleus to the negative electron is less because the number of shielding increases.
- This makes it easier to remove the outer electron and makes the atom more reactive.
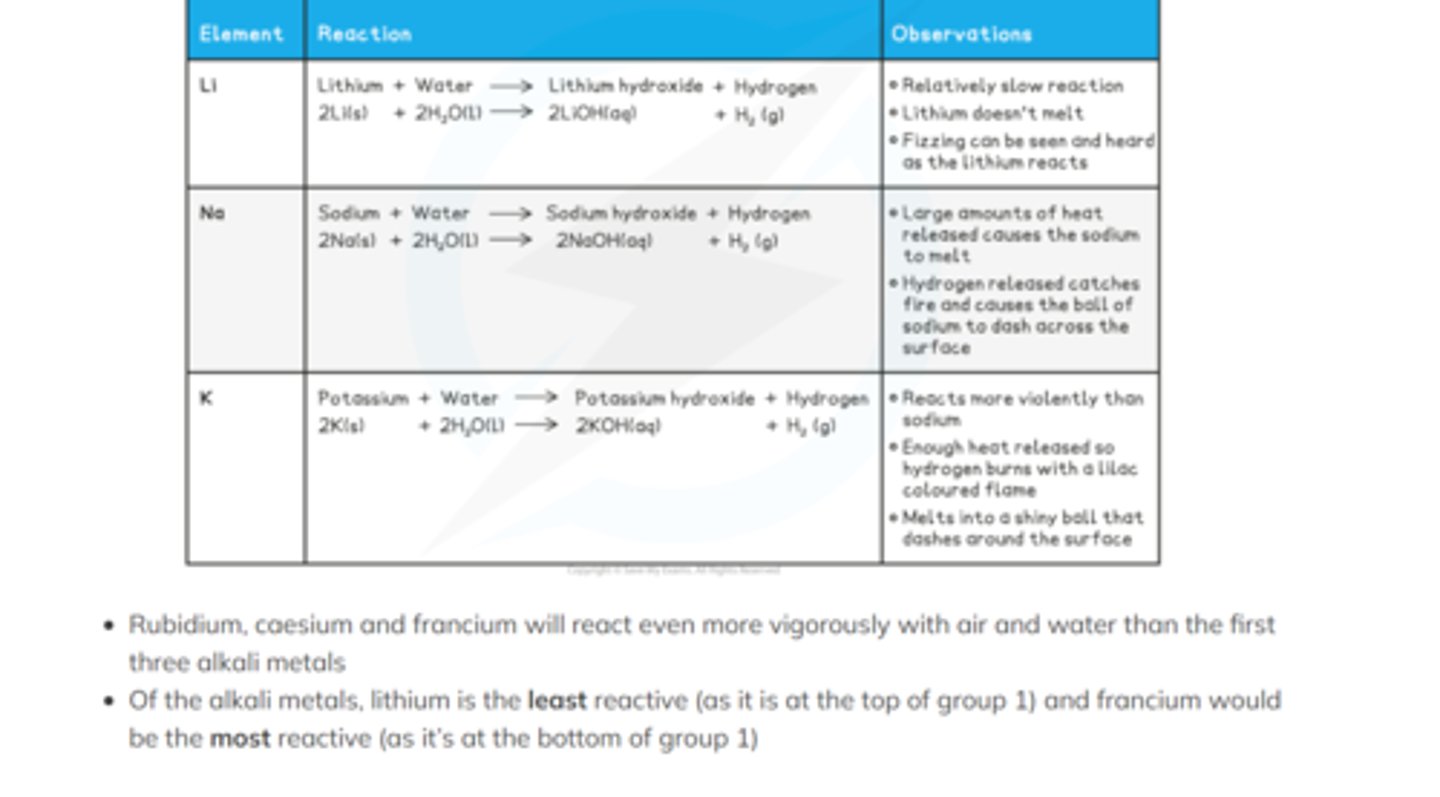
group 1 reactions with water
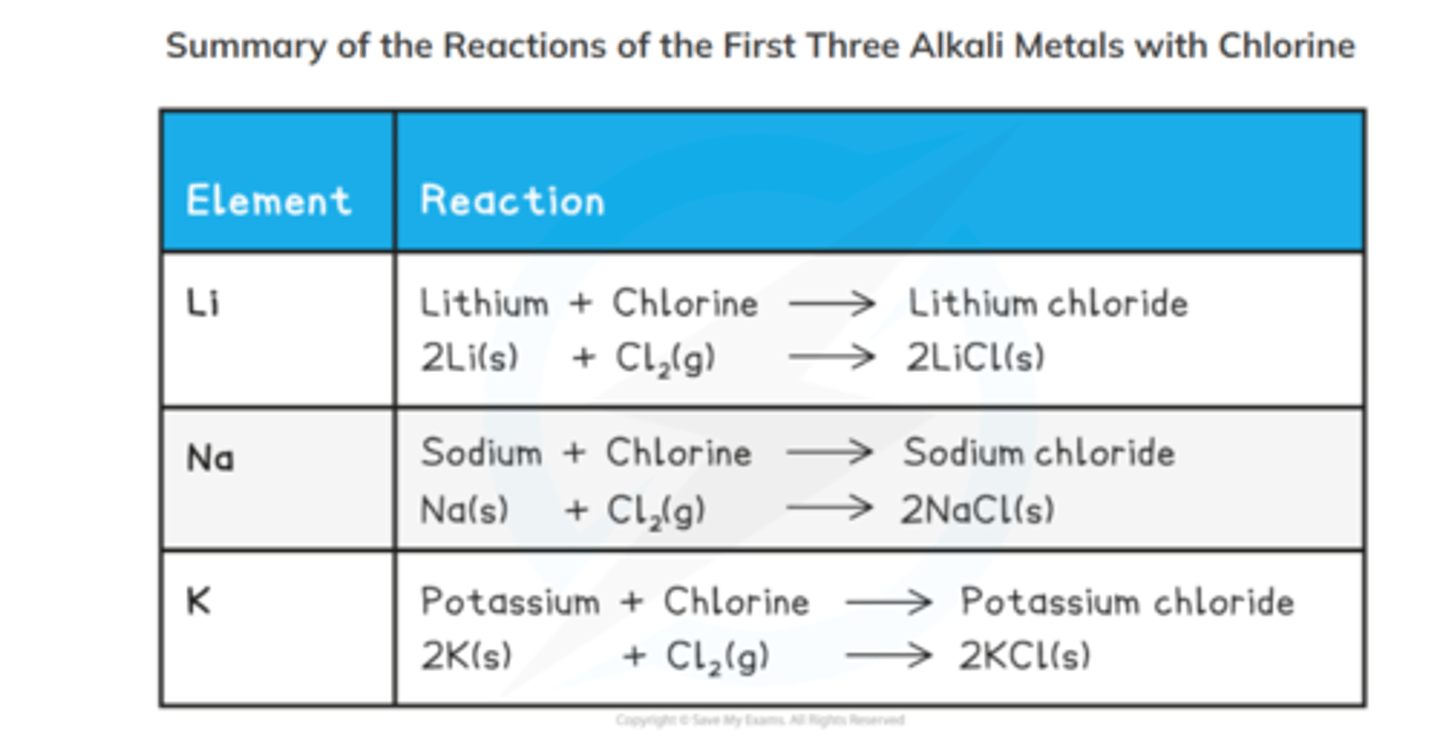
group 1 reactions with chlorine
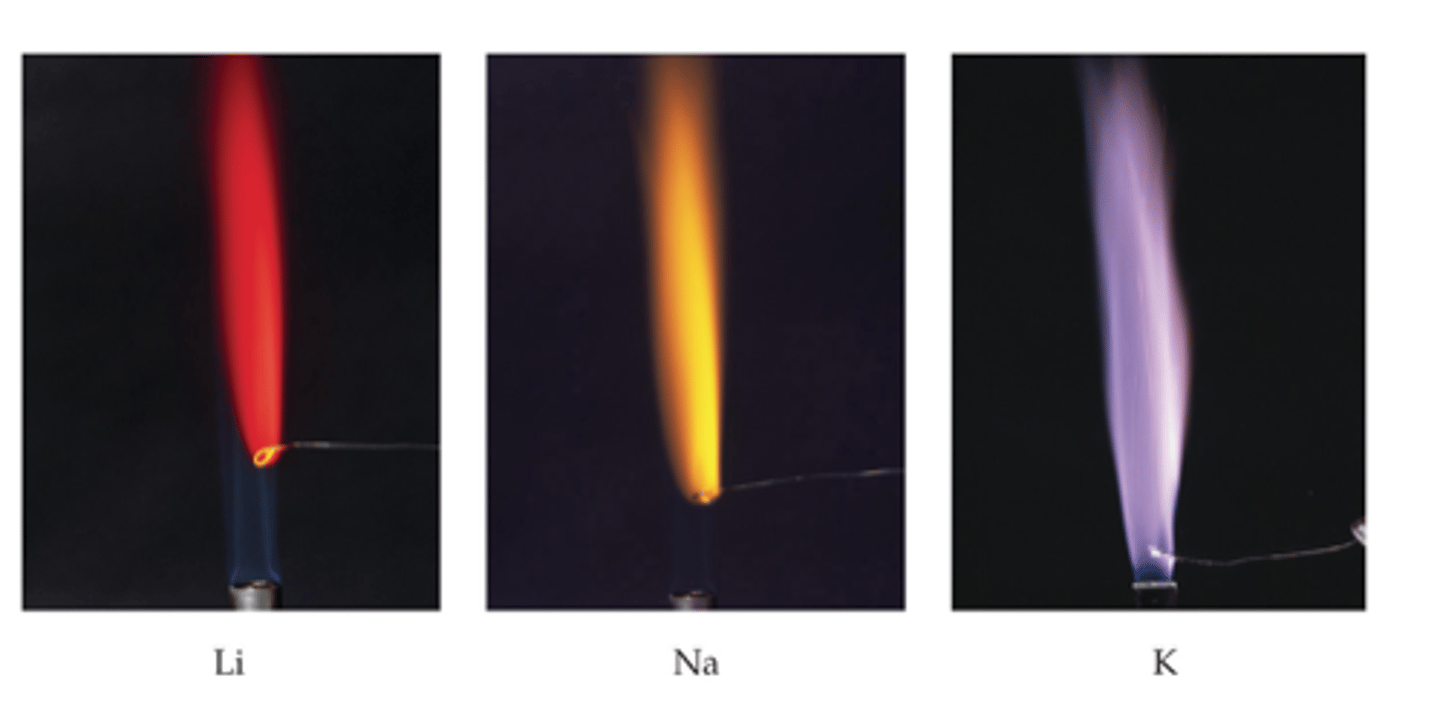
group 1 flame tests
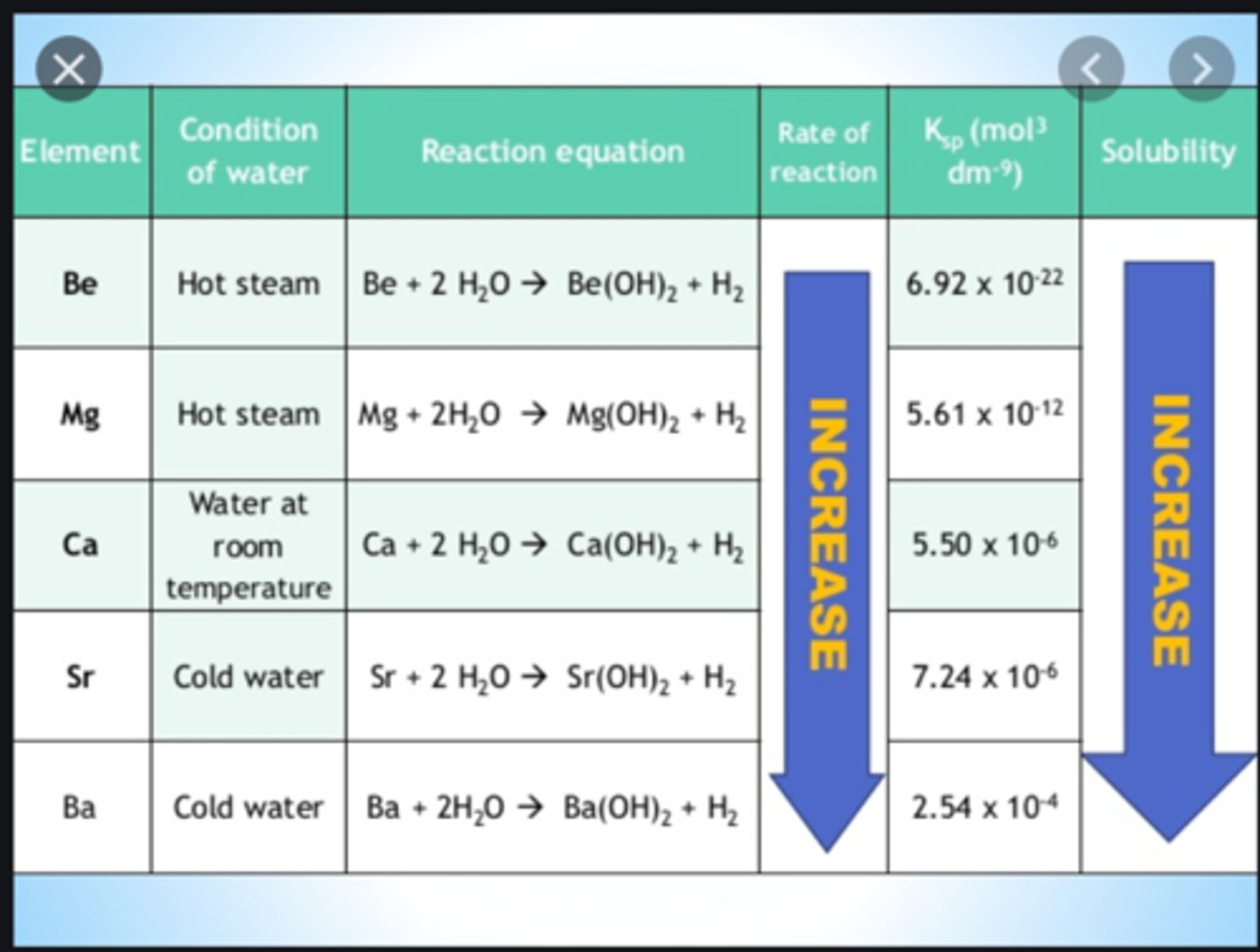
Group 2 reactivity
Increases down the group
increases, decreases, increases
Group 2 trends
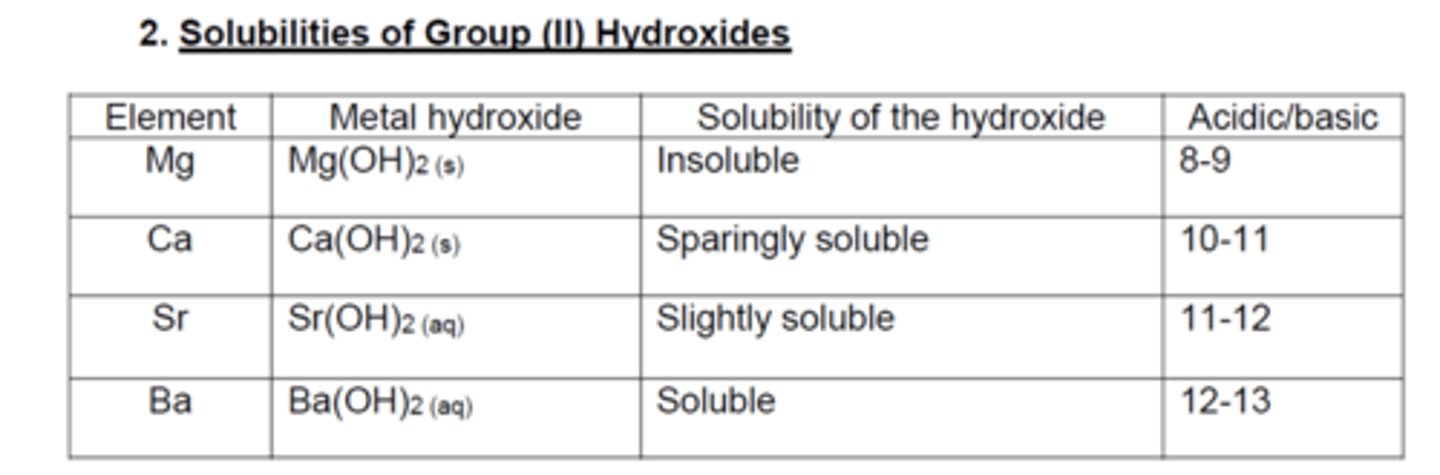
Hydroxides:
solubility ______________ down the group
Sulfates
solubility _________________ down the group
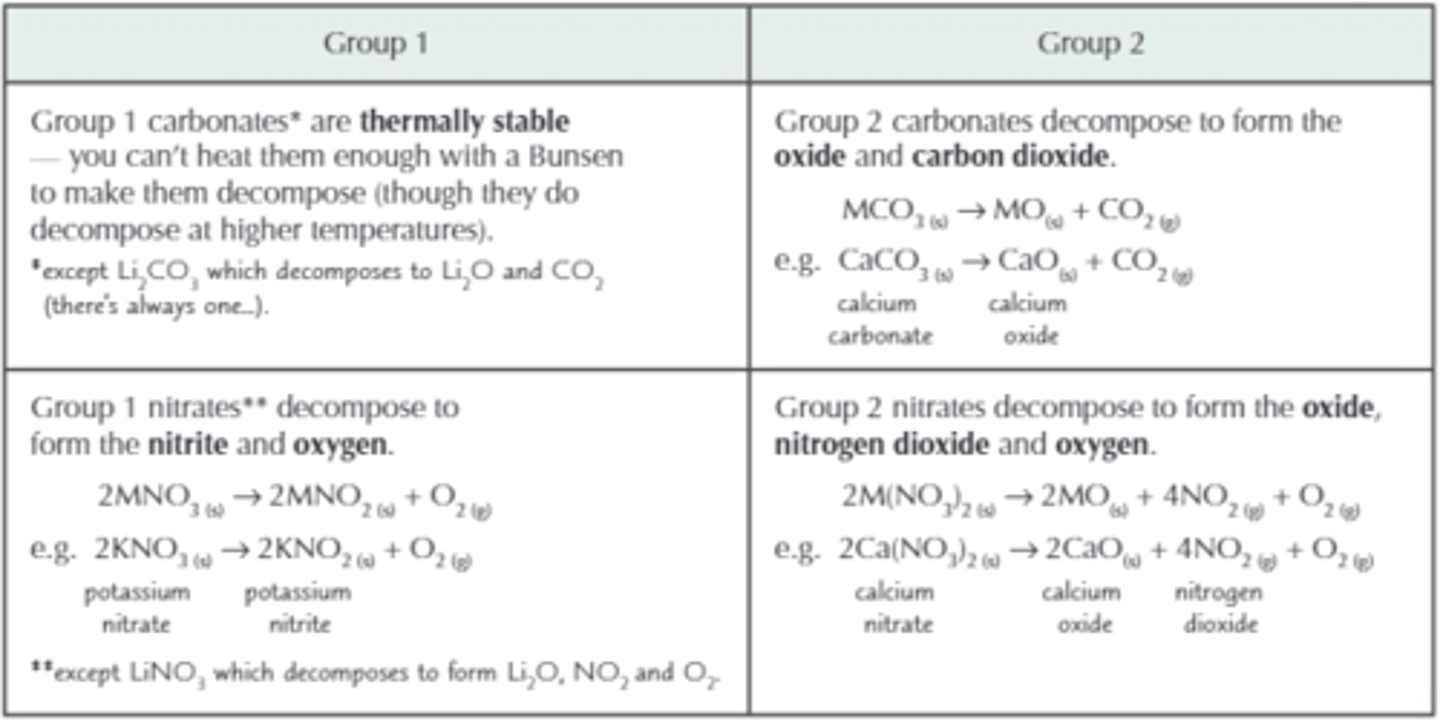
Thermal stability of nitrates and carbonates
_______________ down the group
Group 2 reactions with oxygen
group 2 elements react with oxygen to form a solid, white metal oxide. general formula MO, made up of M2+ ions and O2- ions.
2Ca (s) + O₂ (g) → 2CaO (s)
0 +2 (ox)
0 -2 (red)
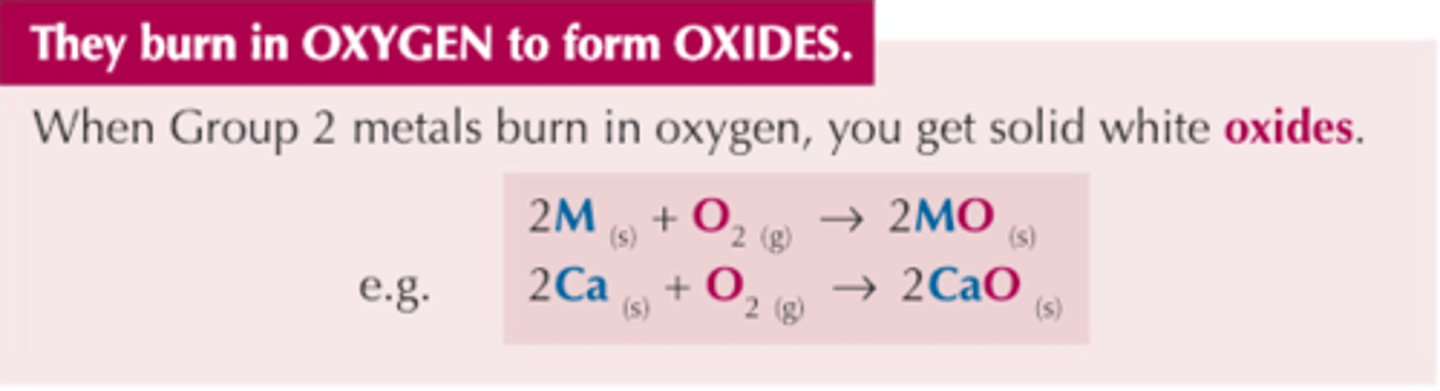
Group 2 reactions with water
Calcium - effervescence, hydroxide turns white, white smoke
Magnesium - burns in steam to produce white magnesium hydroxide and hydrogen gas
Strontium - reacts slowly, the metal sinks and after a short while bubbles of hydrogen are evident stuck to the surface of the metal
Group 2 reactions
metal + water -> metal hydroxide + hydrogen (displacement)
metal + (oxygen chlorine or water) : straightforward addition reactions
metal (oxide or hydroxide ) + dilute acid -> salt + water
(neutralisation)
group 2 flame tests
Calcium - brick red
Strontium - red
Barium - pale green
Thermal stability of nitrates and carbonates
Thermal decomposition
The breaking up of a chemical substance with heat into at least two chemical substances.

Test for ammonium ions
~ Ammonia gas is alkaline so use a damp piece of red litmus paper
~ Add a few drops of aqueous sodium hydroxide and warm the mixture
~ if Litmus paper turns blue then there is ammonia gas produced
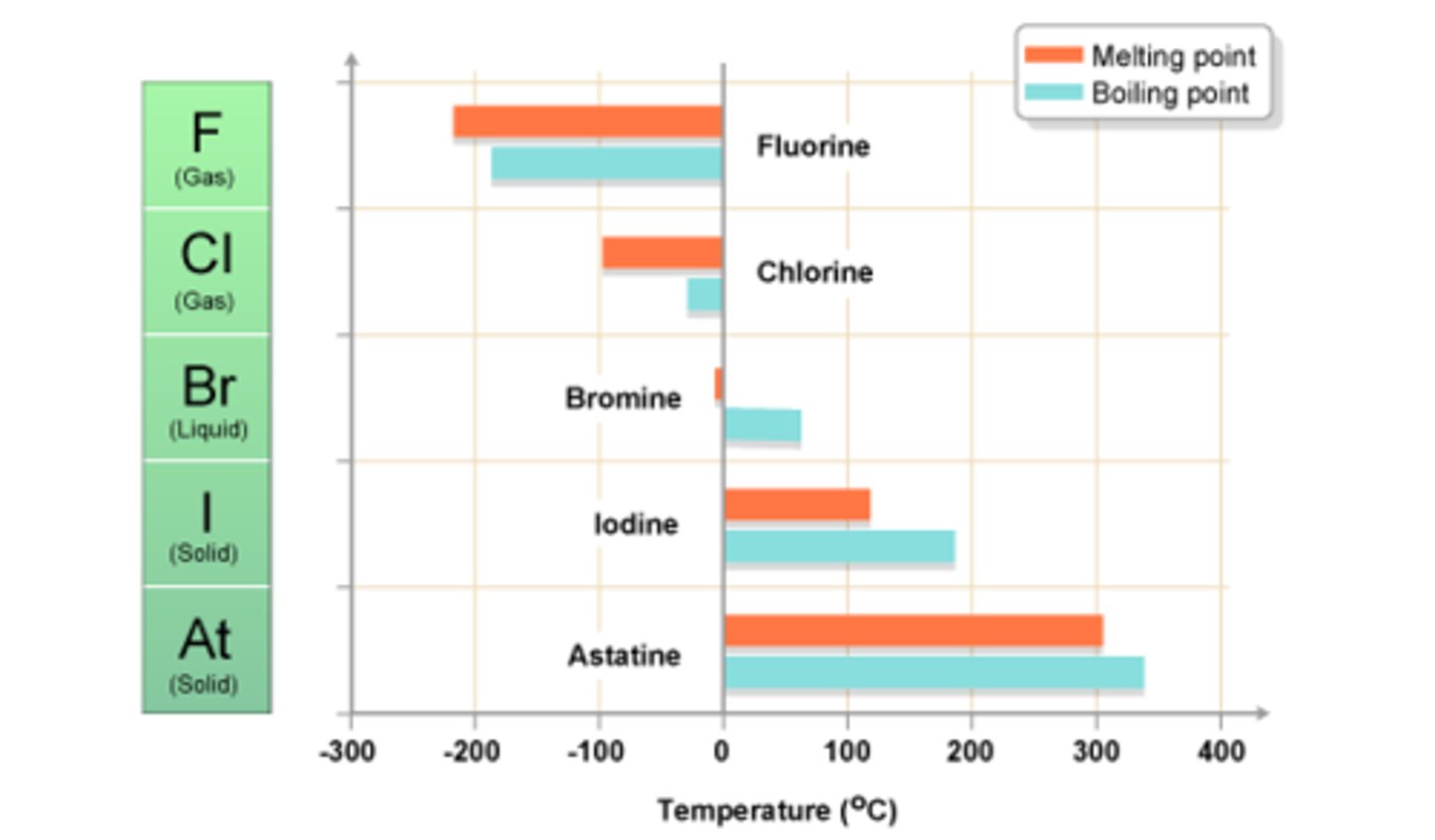
Group 7 melting and boiling points
increase going down the group
this is due to increasing strength of London dispersion forces between the nonpolar diatomic molecules, as the atomic size increases
decreases, decreases, increases
Group 7 (halogen) trends
electronegativity _______________ down the group
reactivity __________________ down the group
boiling point _______________ down the group
Halide displacement reactions
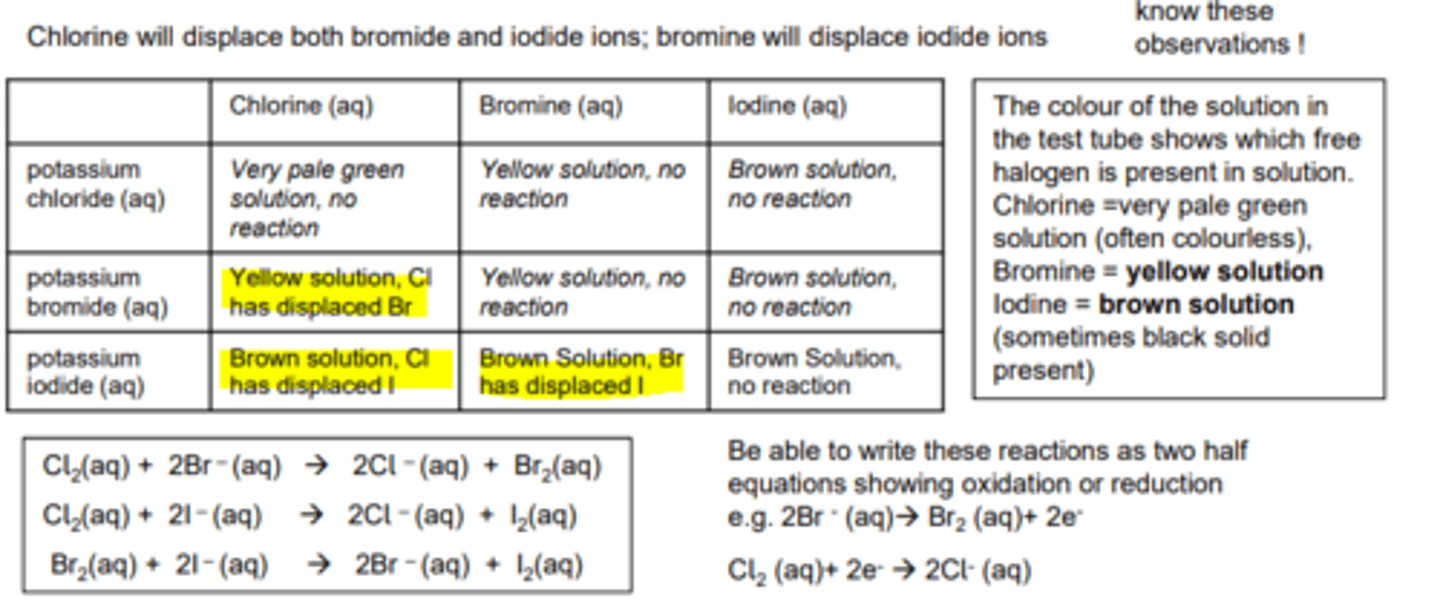
Group 7 reactions
Cl2 + H2O -> HCl + HClO
HClO is used to purify water
chlorine with cold sodium hydroxide
Cl2 + 2NaOH -> NaClO + NaCl + H2O
NaClO is a bleach
any halogen with hot sodium hydroxide
3Cl2 + 6NaOH -> NaClO3 + 5NaCl + 3H2O
Metal + Halogen -> halide salt (simple addition)
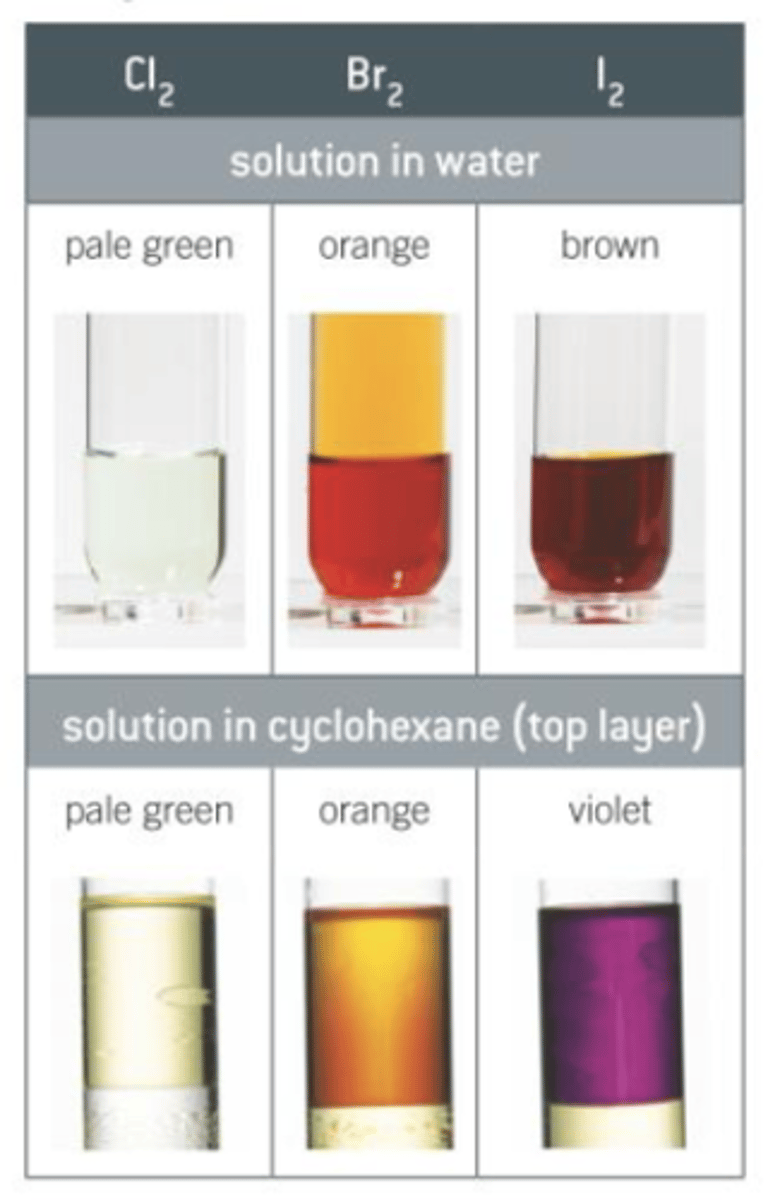
Halogen colours
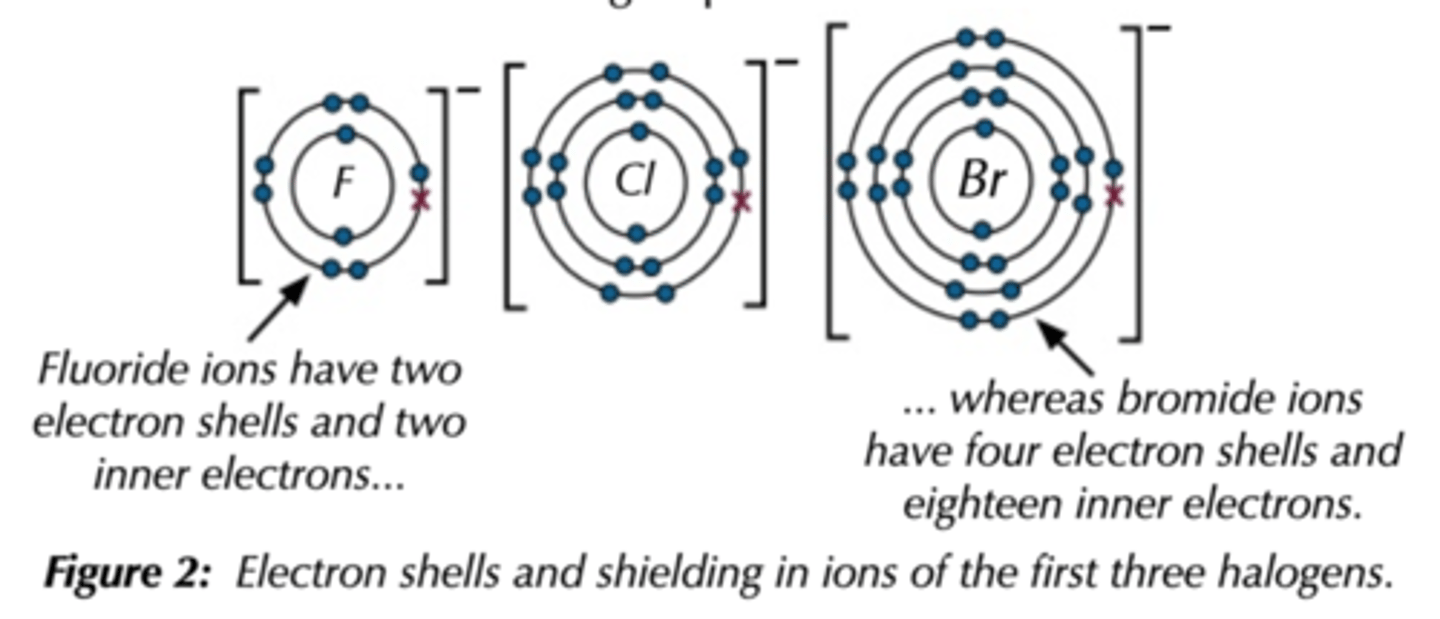
Reducing power of halides
Increases down the group because more shells -> more shielding -> loses electrons more easily
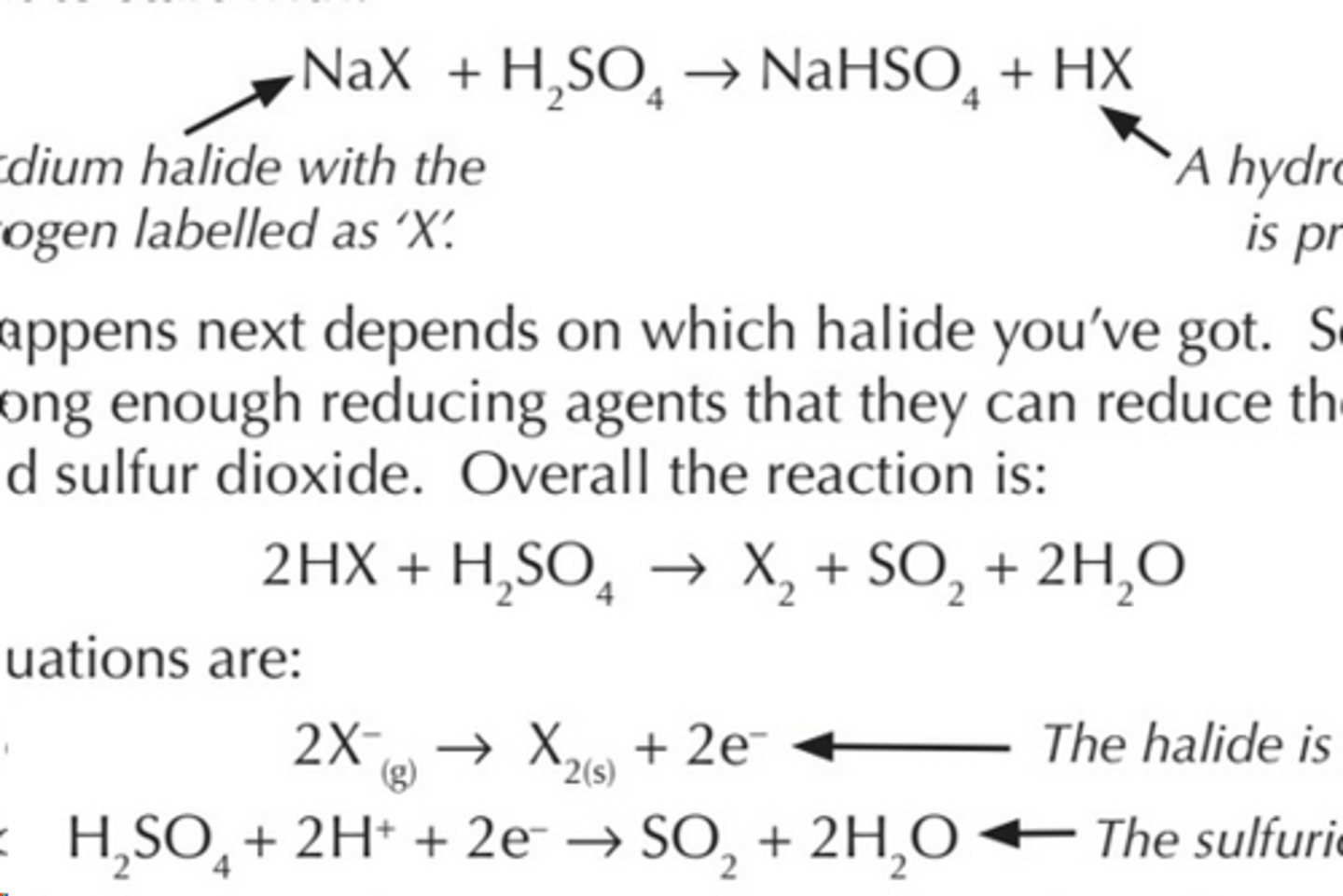
reaction of halides with sulfuric acid
Testing for halide ions
Aqueous Halides react with Aqeuous Silver Ions to Form Precipitates of Silver Halides
Ag+ + X- --> AgX
Chlorine - White
Bromine - Cream
Iodine - Yellow
Add aqueous ammonia to test solubility since colours can be difficult to tell apart
Chlorine - Soluble in dilute NH3
Bromine - Soluble in conc. NH3
Iodine - Insoluble in conc. NH3

Test for sulfate ions
Add dilute hydrochloric acid and then barium chloride solution
if a white precipitate forms, this is a positive test result

Test for carbonate ions
add dilute HCL, it fizzes and the gas evolved turns limewater turns cloudy

standard solution
a solution whose concentration is accurately known
primary standard
A reagent that is pure enough and stable enough to be used directly after weighing.
The entire mass is considered to be pure reagent.
equivalence point
the point at which the two solutions used in a titration are present in chemically equivalent amounts so that there are exactly the right amounts of substances to complete the reaction (no excess)
end point
the point in a titration at which an indicator changes color
titre
the volume added from the burette during a titration
concordant titres
Titres close to each other (usually within 0.2cm³ of each other)
error
the difference between the experimental value and the accepted or correct value
oxoanion
an anion in which an element is bonded to one or more oxygen atoms

half-equation
an equation that describes either reduction (gain of electrons) or oxidation (loss of electrons)
displacement reaction
A reaction in which a more reactive element displaces a less reactive element
Can be used to compare the relative strengths of metals as reducing agents and non-metals as oxidising agents
spectator ions
Ions that do not take part in a chemical reaction and are found in solution both before and after the reaction
ionic reaction
1. balance everything out
2. total ionic equation (split soluble compounds)
3. net ionic equation (no spectator ions)
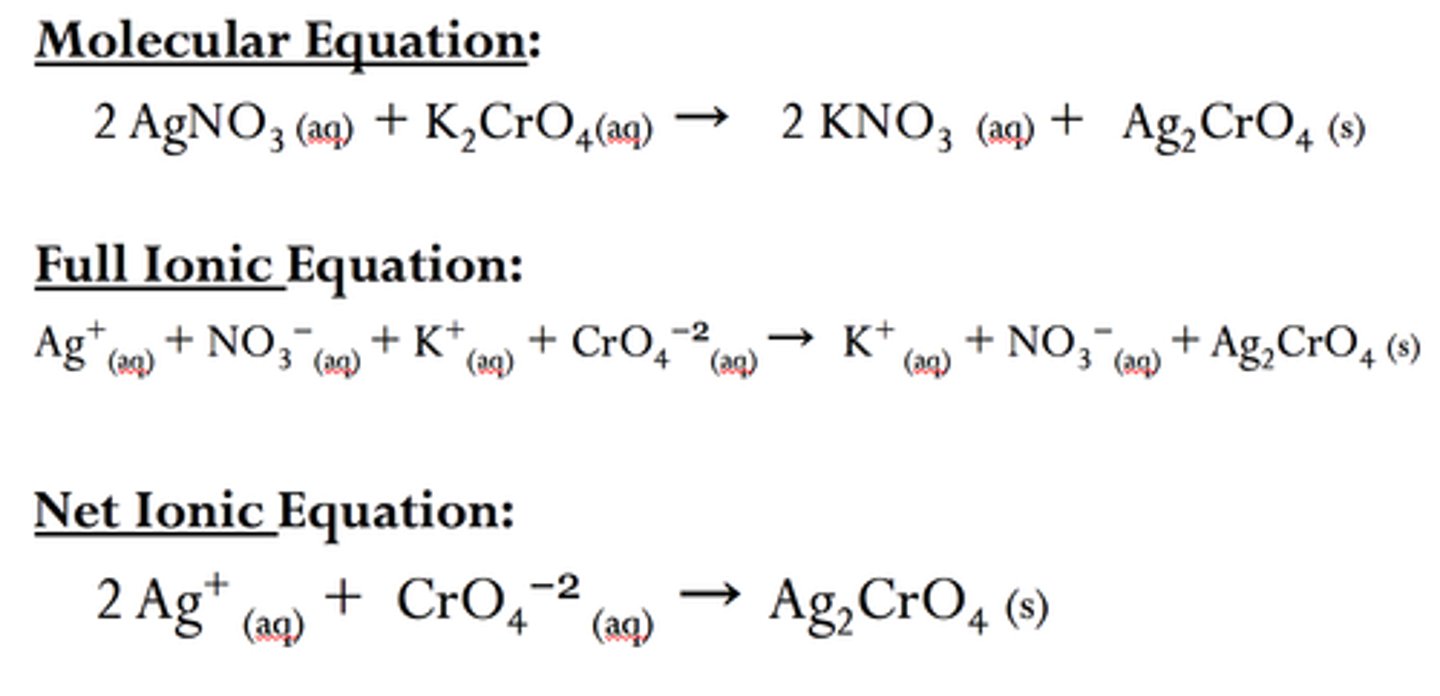
Ionic precipitation reaction
A reaction which produces a solid precipitate on mixing two solutions containing ions
basic oxide
A metal oxide which reacts with acids to form salts and water
thermal stability
a measure of the extent to which a compound decomposes when heated.
Group II are less stable than group I, and e.g. Mg is less stable than Ca, because the smaller the ion and the higher the charge, the greater the polarising power, which distorts the electon cloud and weakens the covalent bonds within the complex anion the metal is bonded to.
chemical kinetics
the area of chemistry that is concerned with reaction rates and reaction mechanisms
rate of reaction
the rate of formation of a product or rate of removal of a reactant
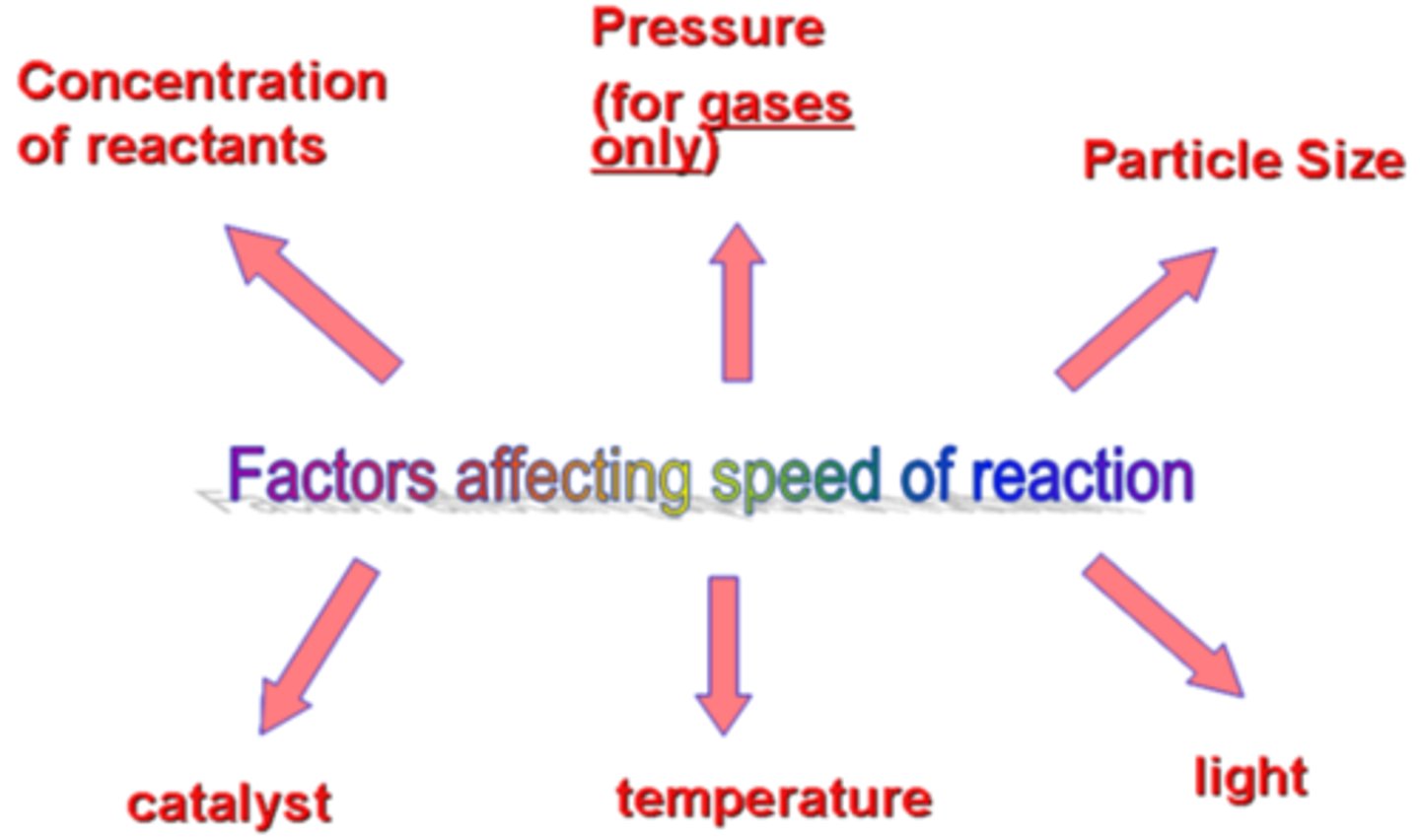
factors affecting rate of reaction
1. temperature
particles have more energy, collide more frequently and with more energy
2. concentration or pressure
more particles in an area = more frequent collisions
3. surface area
smaller pieces = more area for collisions to happen on
4
catalyst
5
light, for photochemical reactions
catalyst
a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
mechanism of reaction
step-by-step description of the process by which reactants are changed into products showing the bonds that break and the new bonds that form as reactants are turned into products
chemical phase
a physically distinct form of matter, such as solid liquid gas
When studying reactions, immiscible liquids are treated as separate phases since a reaction can only occur at the boundary layer between them, not in the bulk
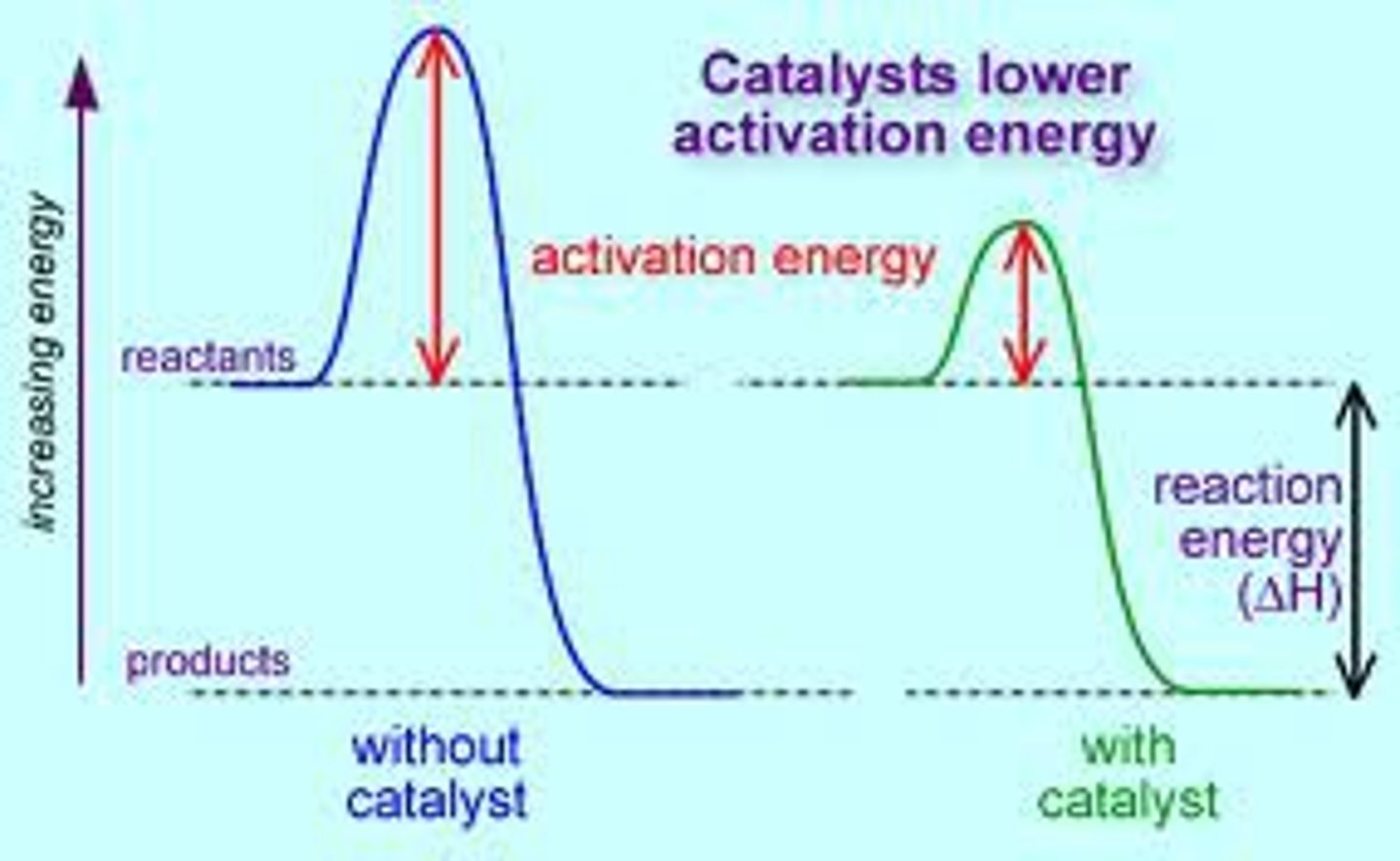
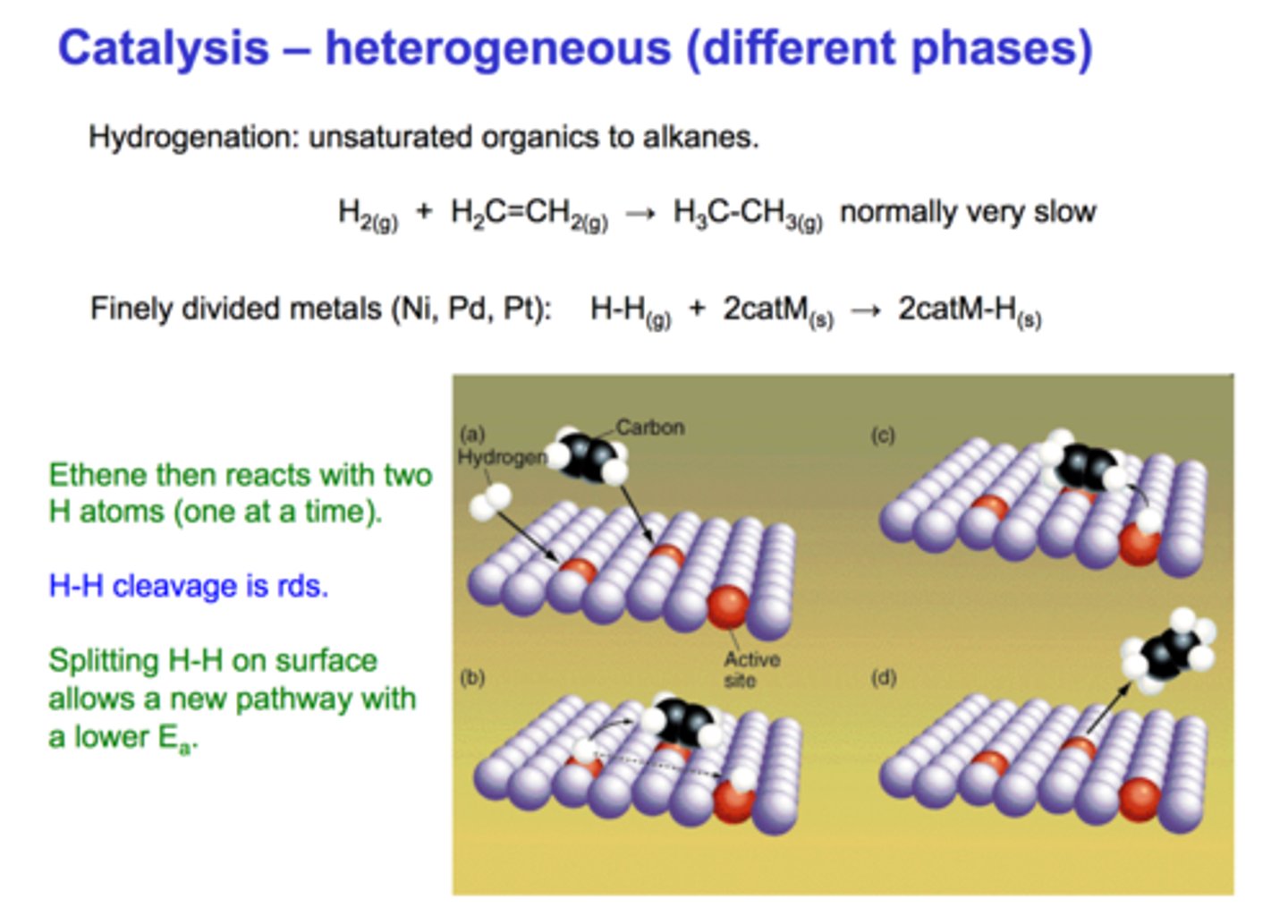
how catalysts work
a catalyst provides an alternative pathway for a reaction
This has a lower activation energy than the uncatalysed reaction
A greater proportion of molecules have an energy >= the activation energy of the catalysed reaction
So a greater fraction of the collisions are successful
(compare with Maxwell-Boltzmann distribution)
(they don't just "lower the activation energy")
equilibrium position
Catalysts don't change the equilibrium _________________. They do affect the ___________ at which equilibrium is approached.
heterogeneous catalyst
A catalyst that is in a different phase from that of the reactant substances.
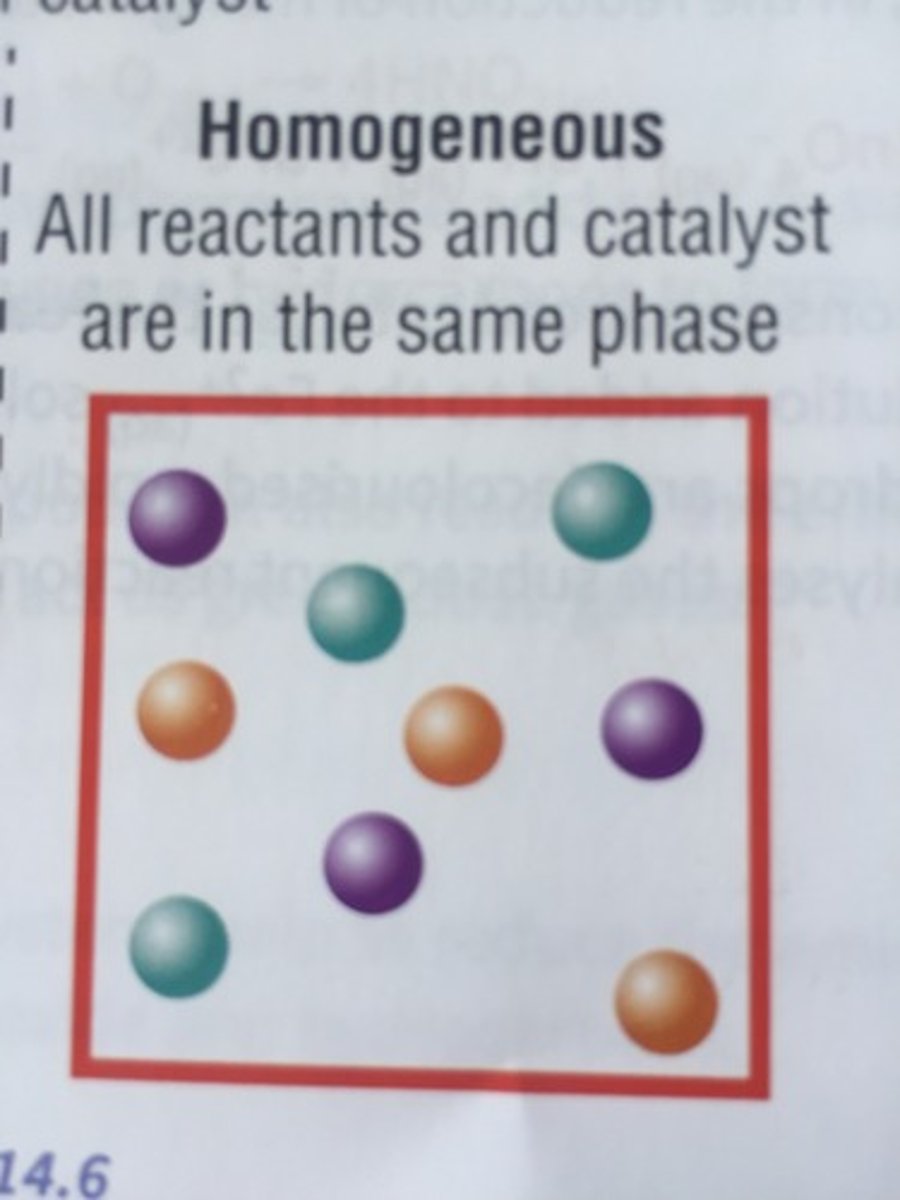
homogeneous catalyst
a catalyst that is in the same phase as all the reactants and products in a reaction system
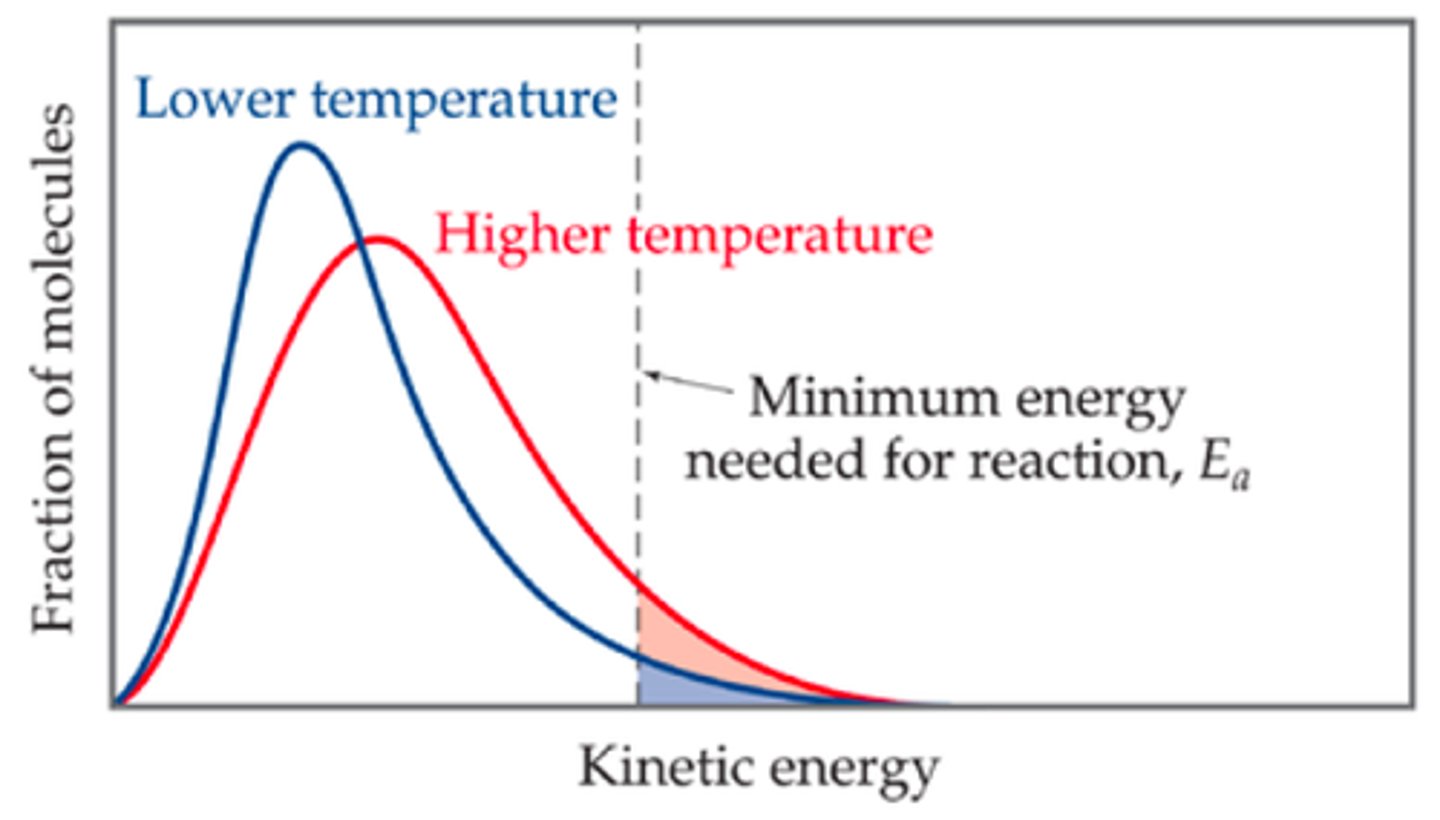
Maxwell-Boltzmann distribution
The distribution of energies (or equivalently, speeds) of the molecules in an ideal gas
It is actually the chi distribution with 3 degrees of freedom (one for each space dimension) - so it's a Root Mean Square of three normals.
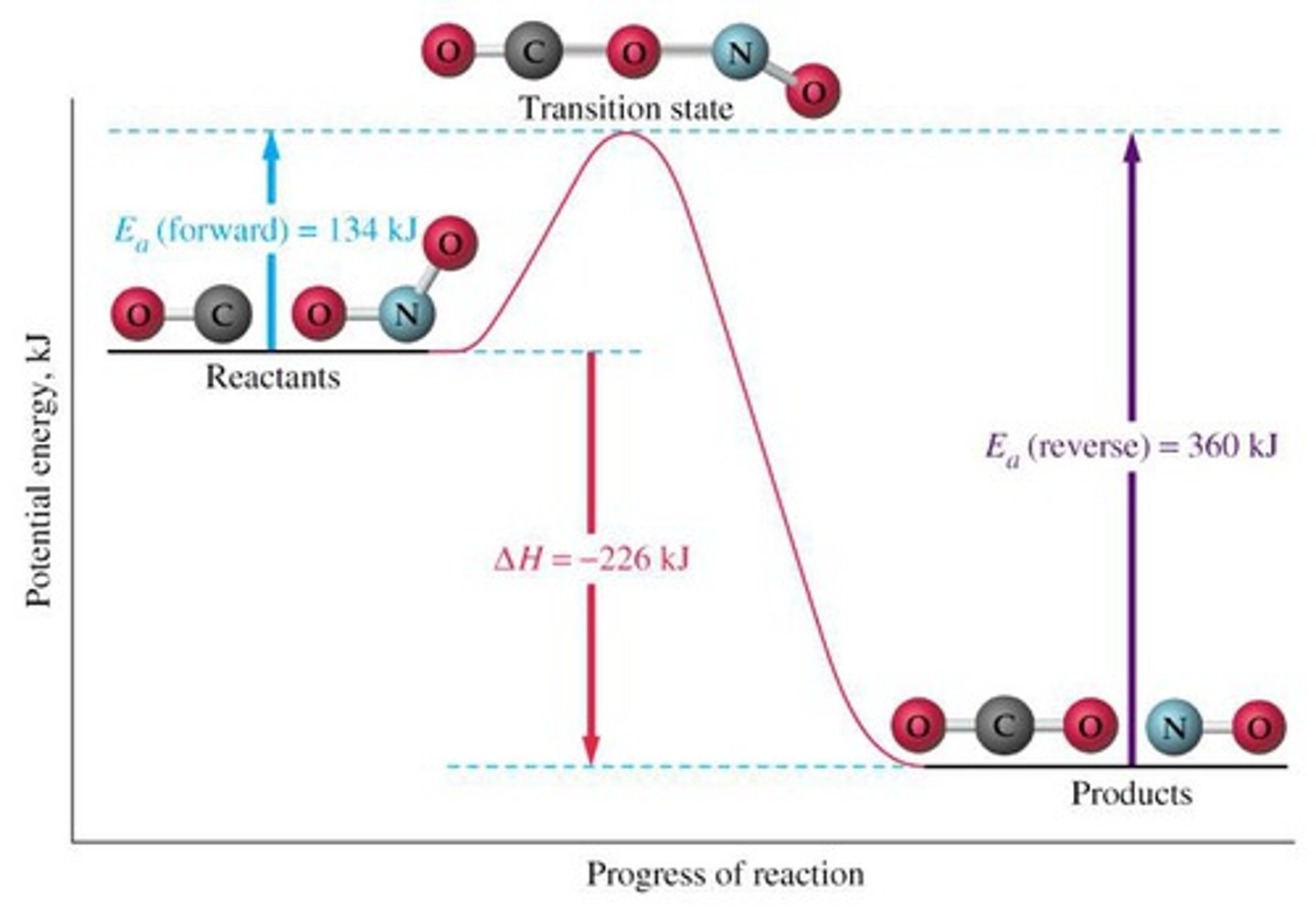
activation energy
the minimum amount of energy required to start a chemical reaction
the height of the energy barrier separating reactants and products
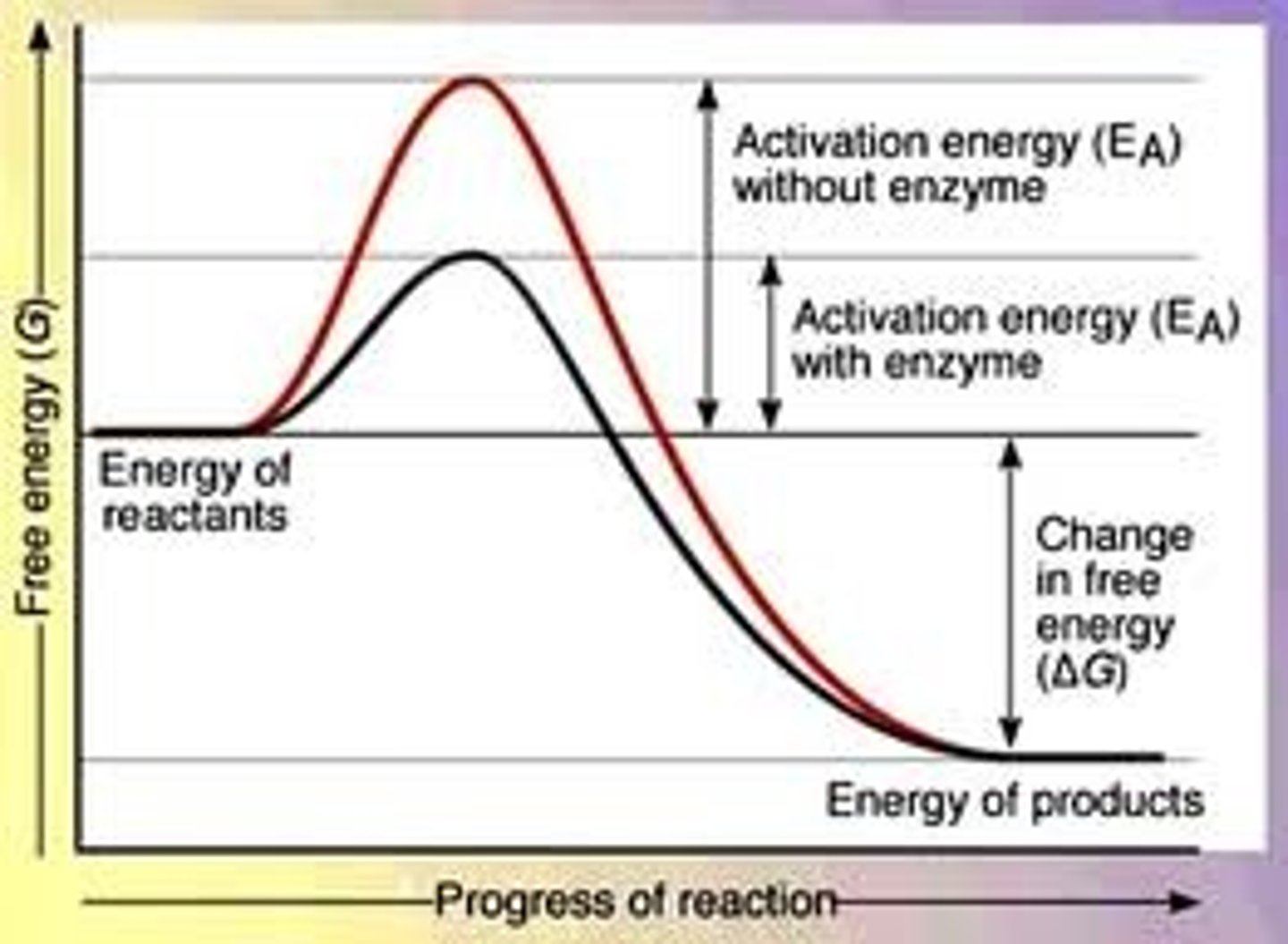
reaction profile
a diagram showing how the total enthalpy of the atoms and molecules changes during the course of a reaction from reactants to products
Collision theory
For a reaction to occur, the particles:
a) must collide
b) must collide with the appropriate orientation
c) must collide with sufficient energy.
it is important to talk about frequency of collisions and proportion of collisions with sufficient energy and suitable orientation
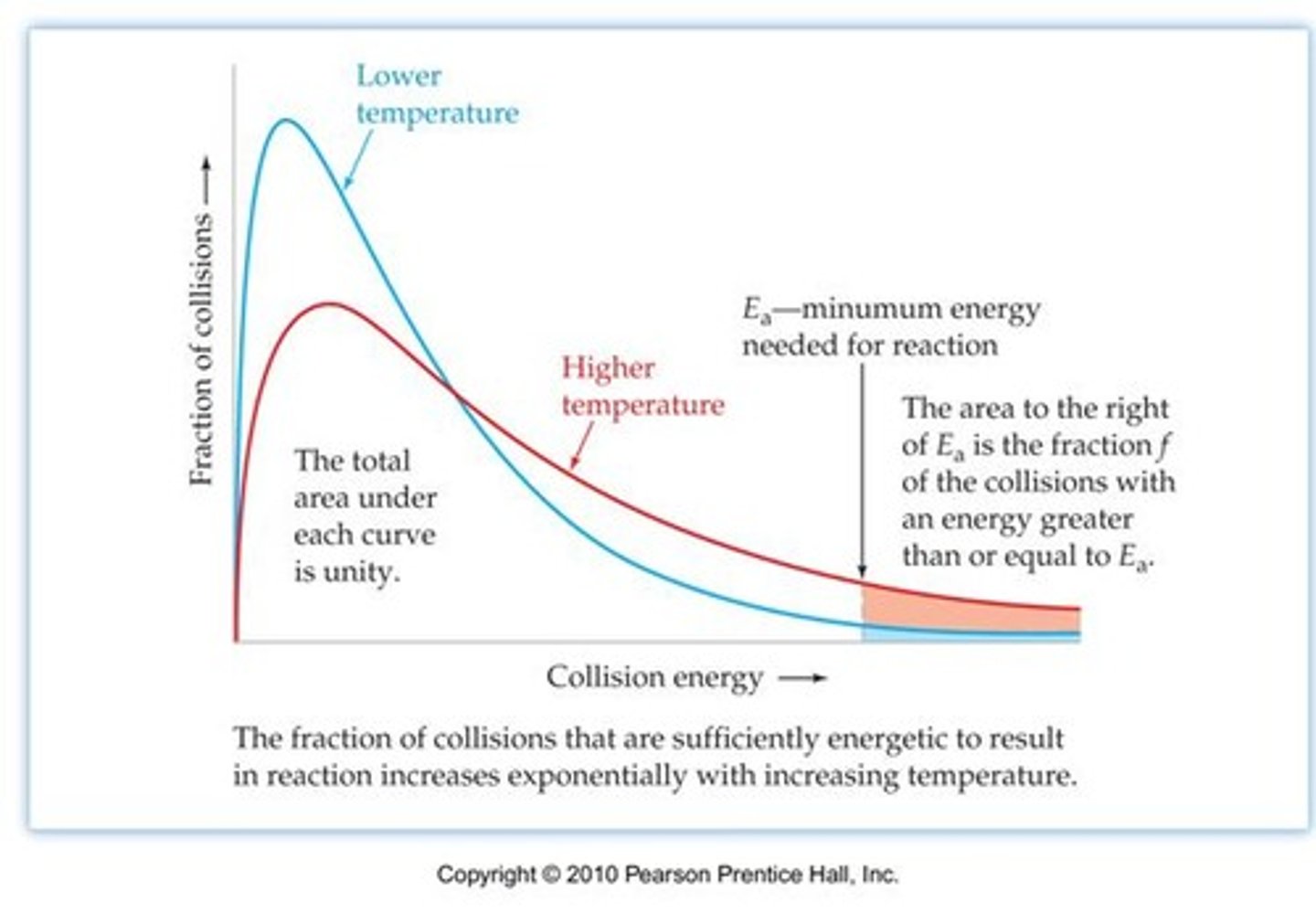
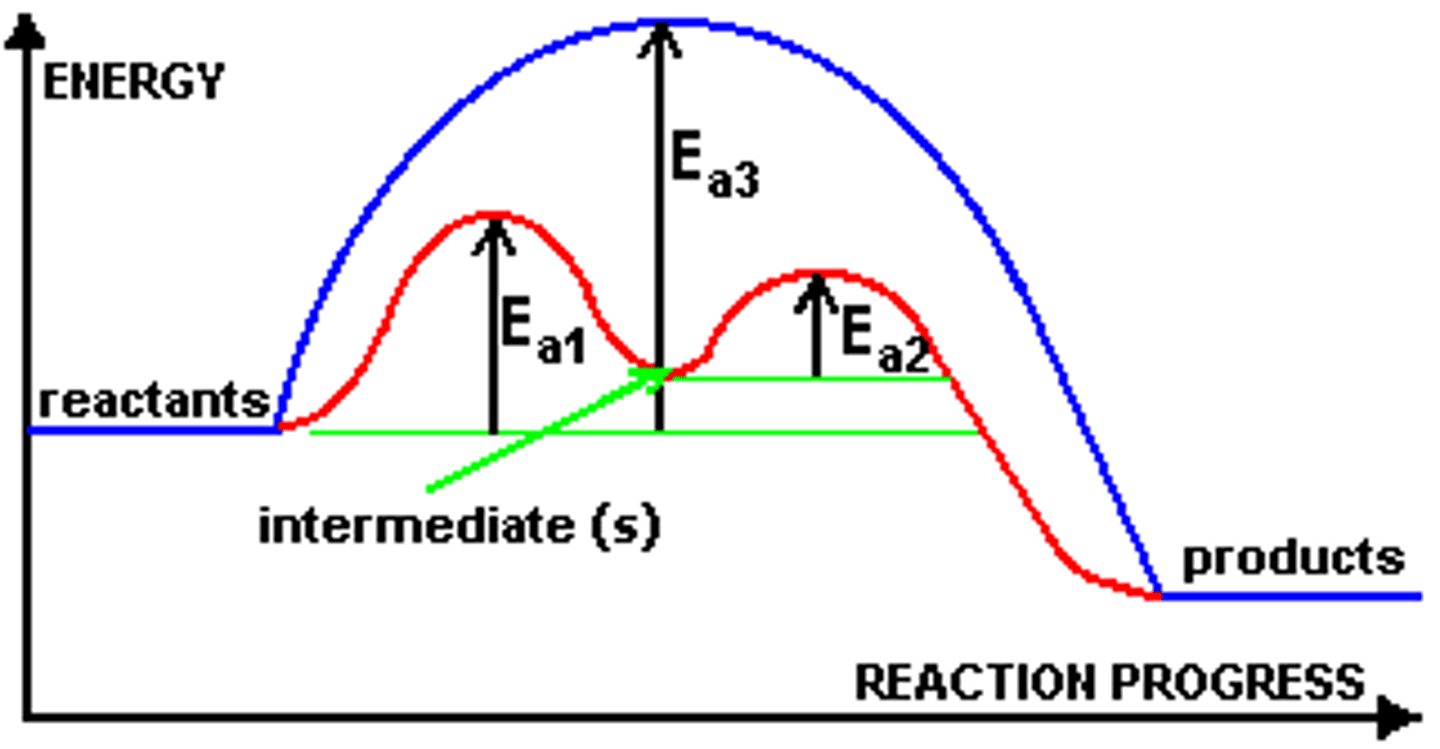
transition state
a high-energy intermediate state of the reactants during a chemical reaction that must be achieved for the reaction to proceed
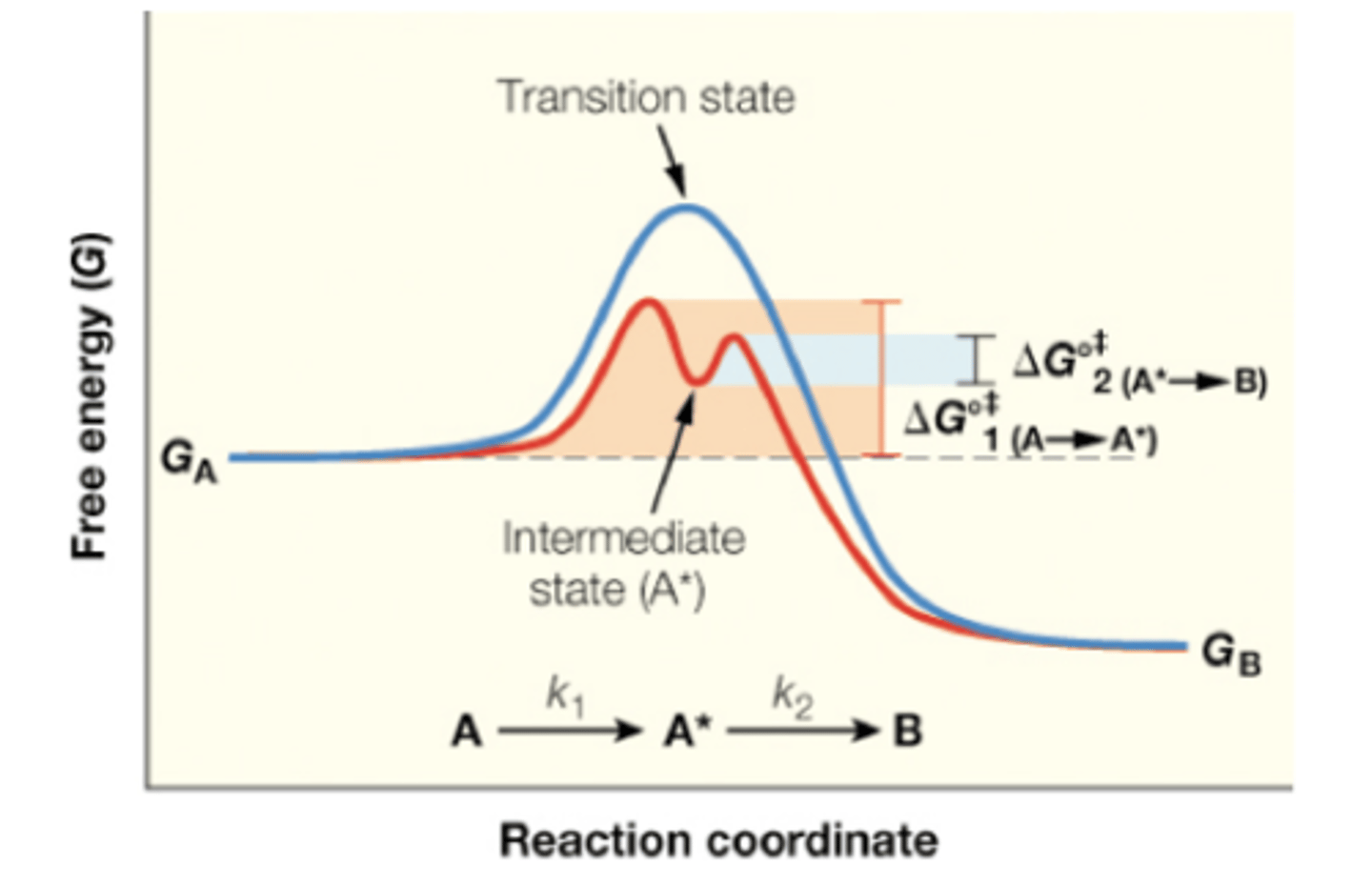
intermediate in reactions
atoms or molecules which do not appear in the balanced equation but which are formed during one step then used up in the next step
steric factor
the factor (always less than 1) that reflects the fraction of collisions with orientations that can produce a chemical reaction.
calculated as the ratio between the experimental rate constant and the one predicted by collision theory
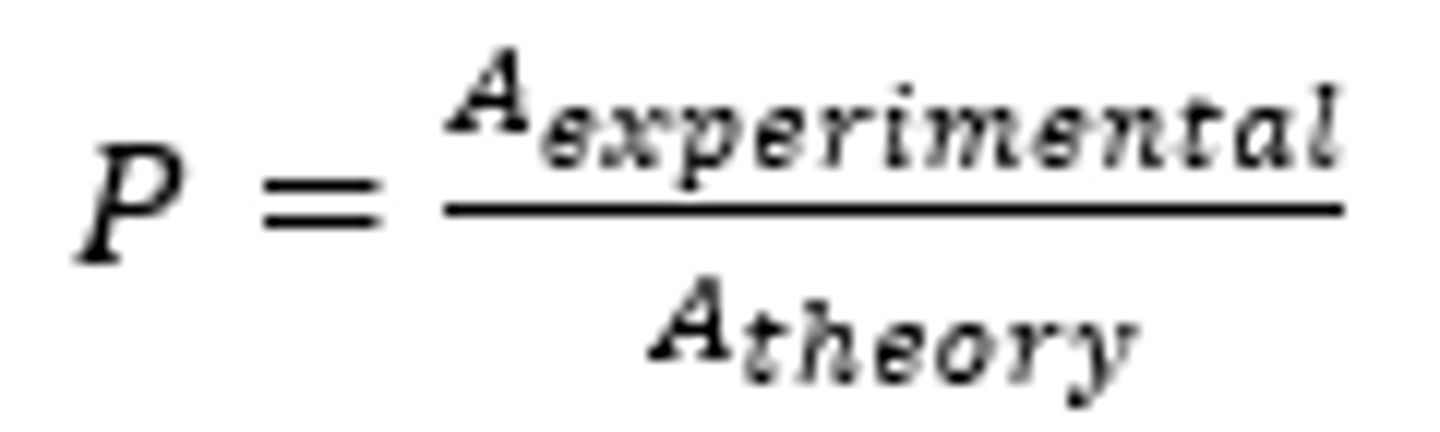
steric hindrance
the slowing of a chemical reaction due to large groups within the molecule getting in the way of the attaching species
reversible change
a process which can be reversed by altering the conditions
dynamic equilibrium
Substances transition between the reactants and products at equal rates, meaning there is no net change.
The forward and backward reactions continue, but at equal rates, so there is no overall change.
At a molecular level there is constant change.