Midterm review
1/45
Earn XP
Description and Tags
Chem
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
g
Unit used for Mass
L
unit used for volume
Unit used for energy
J
Unit used for temperature
C or K
Unit used for length
M or ft
time
seconds mins hours
Density
the measure of how much mass is packed into a specific volume
Never
leading 0s?
Always
middle 0s?
sometimes, yes if there is a decimal, no if not
trailing zeros
If it is odd
it goes up
if its even
it stays
when you add or subtract you go based off of
the least to the Right of the decimal
when you multiply or divide you go based off
the least total numbers
what is matter?
anything in the universe that has mass (takes up space) and volume (occupies space)
light
which of the following has no matter?
Calcium
which of the following is an element
steel
which one of these is homogeneous? the rest are heterogeneous
solvent
the liquid substance
solutes
the solid substance
the reactant side
the left side
the product side?
the right side
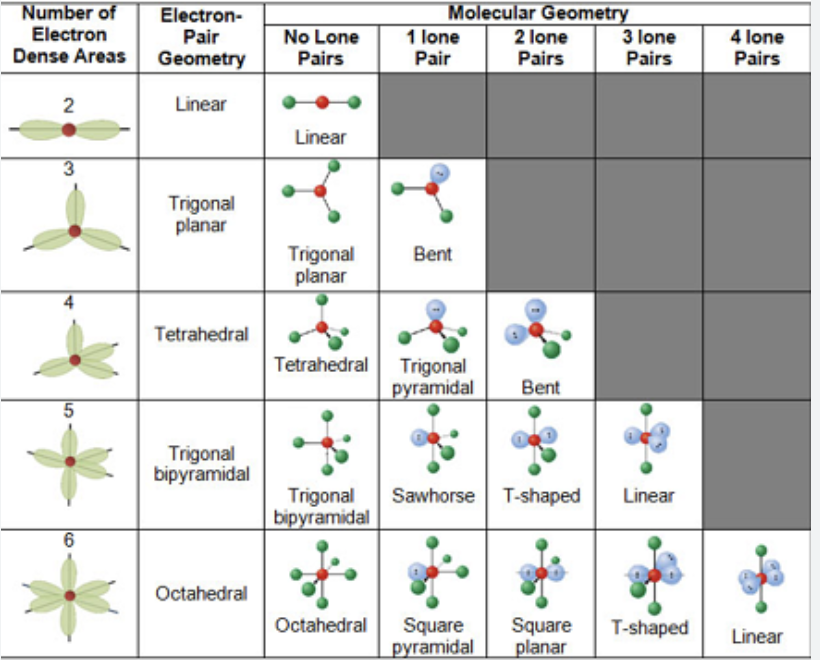
A+2B→ AB2 is what type of reaction
Synthesis
AB2→A+2B is what type of reaction?
Decomposition
CH4+O2→CO2+HO is what type of reaction?
Combustion
AB+C→ CB+A is what type of reaction
Single replacement
AB+CD→AD+CB is what type of reaction?
Double replacement
what is an empirical formula?
A formula where any molecular formula is reduced (divide by greatest common factor)
how to find percent composition
divide the mass of one element in a compound by the total mass of the compound, then multiply by 100%
Ionic
Metal nonmetal
Covalent
Nonmetal nonmetal
remember to write a formula to find the charges on the periodic table and swap them to find their charges
ok
Protons charge mass and location
positive, 1.67 ×10^-27 kg, inside the nucleus
what is an amu
a tiny unit of mass used for atoms and molecules,
what are orbitals
3D regions around an atom's nucleus that describe the probability of finding an electron
Neutron charge mass and location
no charge, 1.67 × 10^-27 kg, inside the nucleus
Electron charge mass and location
Negative, 9.1 × 10^-31 kg, orbiting the nucleus
if atoms are neutral then the number of protons ____ the number of electrons because ____
the same as, the positive and negative charges balance perfectly
how can you tell how many protons an element has?
the atomic number
what are positive ions called and how are they formed
cations, and they form when a neutral atom loses one or more electrons,
what are negative ions called and how are they formed
anions, and they form when a neutral atom gains one or more extra electrons, resulting in more electrons (negative charge) than protons (positive charge)
isotopes
a version of a chemical element that has the same number of protons but a different number of neutrons
isotopes have in common
the same number of protons (same atomic number) and electrons
how are isotopes different
differ in their number of neutrons
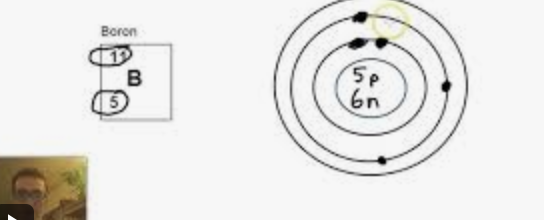
how to draw a bohr diagram
ok