Covalent bonding and simple molecular substances
1/11
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What are covalent bonds?
When non-metal atoms bond together. They share a pair of electrons to make covalent bonds.
Why are covalent bonds strong?
The positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces.
How do you use a dot and cross diagram to draw covalent bonds?
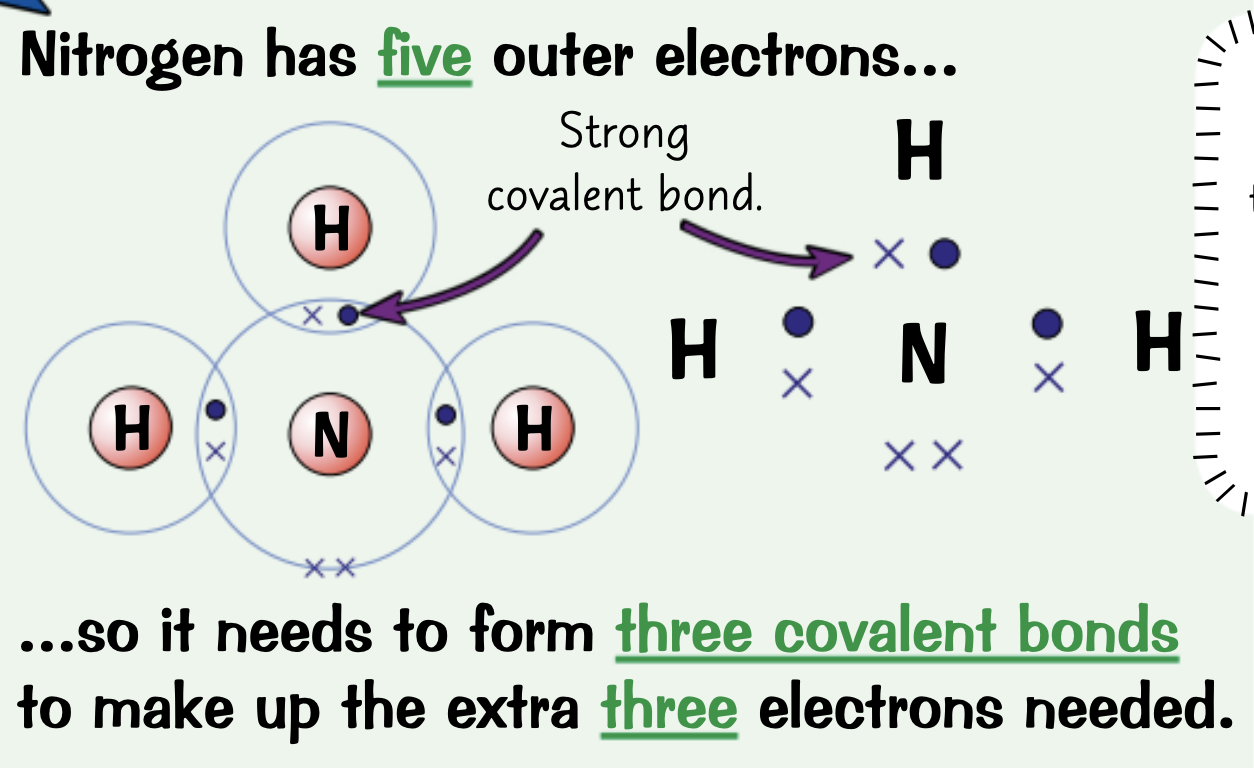
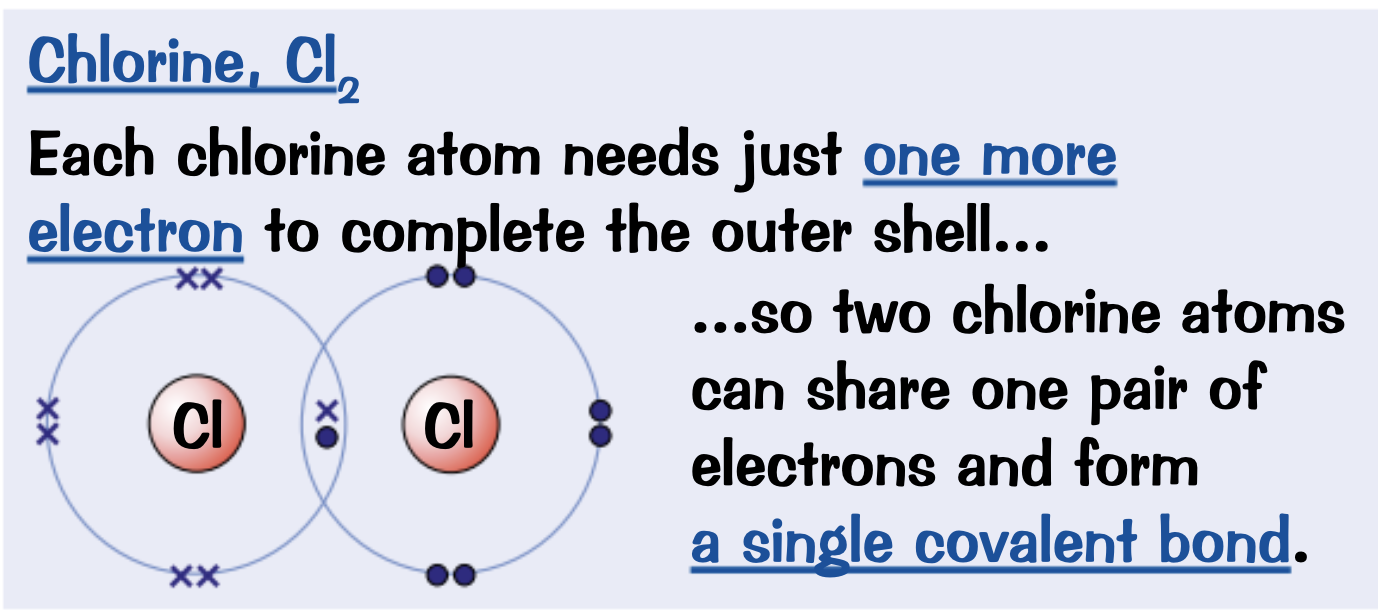
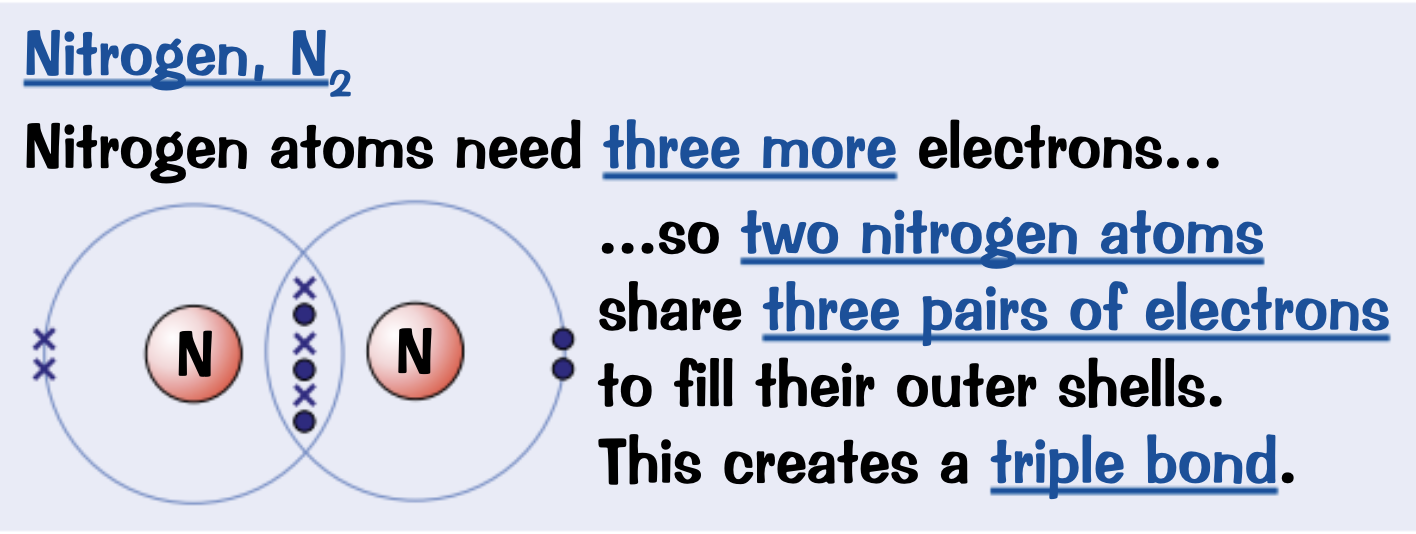
Electrons drawn in the overlap between outer orbitals of the two atoms are shared between those atoms.
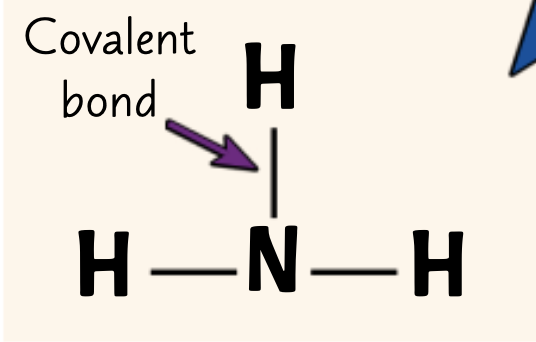
How are covalent bonds shown in displayed formulae?
Draw a dot and cross diagram for Hydrogen, H2

Draw a dot and cross diagram for Chlorine. Cl2
Draw a dot and cross diagram for Oxygen, O2

Draw a dot and cross diagram for Nitrogen, N2
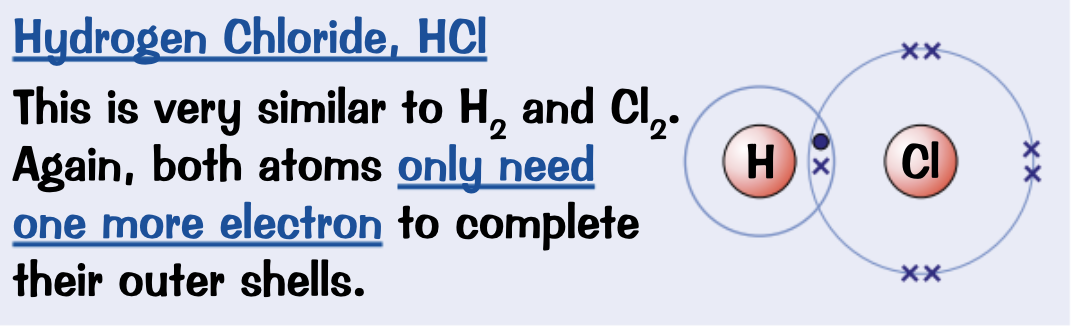
Draw a dot and cross diagram for Water, H2O
Draw a dot and cross diagram for
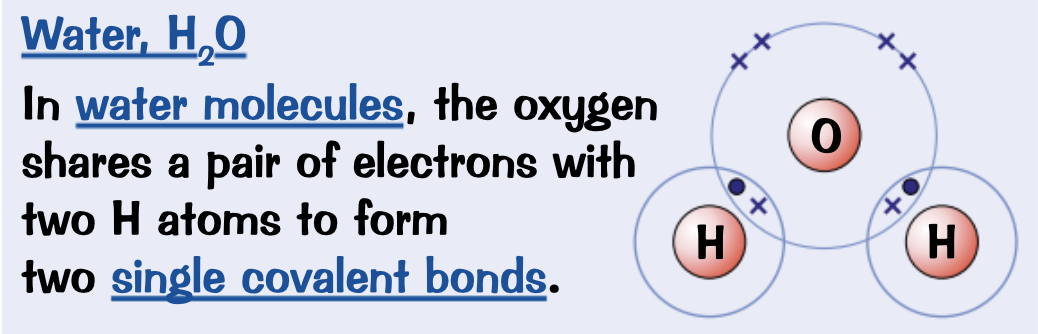
Draw a dot and cross diagram for Methane, CH4
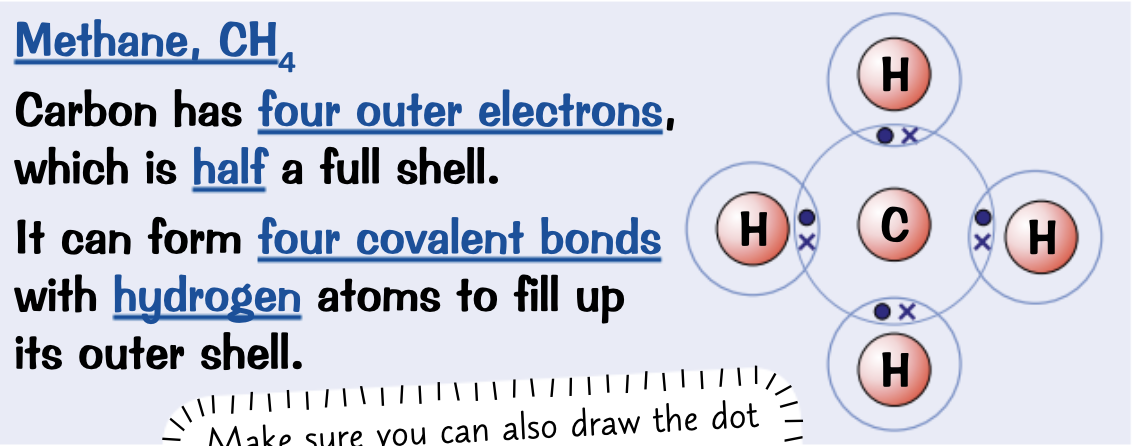
Draw a dot and cross diagram for Hydrogen chloride, HCl
What are the properties of simple molecular substances?
Substances containing covalent bonds usually contain simple molecular structures
The atoms within the molecule are held together by very strong covalent bonds, but the forces of attraction between these molecules are weak
Low bpt and met because the intermolecular forces are easy to break and you don’t need to break the covalent bonds
Mostly gases and liquids at room temp
As the molecules get bigger, the strength of the intermolecular forces increases, so more energy is needed to break them so the mat and bpt increases
Don’t conduct electricity because they have no charge and no free electrons