2.1.2 Biological Molecules
1/65
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
What is the structure of water?
Water consists of 2 hydrogen atoms covalently bonded to one oxygen atom
How does hydrogen bonding occur in water?
Each hydrogen shares a pair of electrons with the oxygen (covalent bonding).
Oxygen has a greater affinity for electrons than hydrogen, so it ‘pulls’ the electrons closer
What are the properties of water?
High specific heat capacity
High latent heat of vaporisation
Surface tension
A solvent
An insulator
Higher density than ice
A transport medium
What is surface tension and how could it benefit organisms?
The hydrogen bonds mean the water is ‘sticky’ by forces of adhesion & cohesion.
Molecules in the bulk of liquid are not affected by molecules above, therefore ‘pull’ more strongly to form a stretched membrane → a habitat can exist on water (pond skaters)
How can being a solvent be beneficial for organisms?
dissolves most organic & inorganic substances
needed for biochemical reactions
removes excretory products e.g. urea
in plants, root hairs absorb mineral salts present in soil
How can being an insulator be beneficial for organisms?
ice is less dense than water, therefore floats above it → insulates the water below the surface of ponds & lakes
water won’t freeze, so aquatic organisms can survive
How can a high latent heat of vaporisation be beneficial for organisms?
loss of heat through vaporisation → sweating, which helps us cool down & prevents overheating
How does being a transport medium be beneficial for organisms?
human blood plasma consists mainly of water (90%)
carries many dissolved substances like excretory waste, hormones & gases around the body
in plants, sugar & mineral salts are transported in solution in vascular bundles
What are carbohydrates?
A group of substances used as both energy sources and structural materials in organisms
contains C, H & O
What are monosaccharides?
simple sugar molecules (monomers)
all are reducing sugars
general formula = (CH2O)n
What is the difference between a hexose monosaccharide and a pentose monosaccharide?
hexose contains 6 carbon atoms
pentose contains 5 carbon atoms
What are the properties of glucose?
hexose sugar
highly soluble
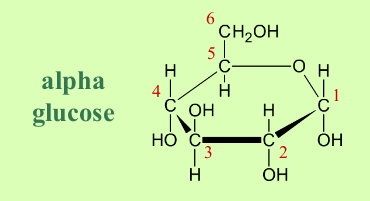
What is the structure of alpha glucose?
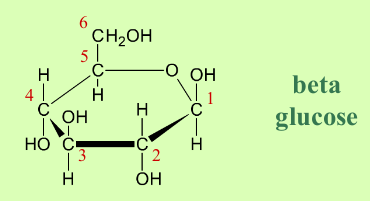
What is the structure of beta glucose?
What is the difference between alpha and beta glucose?
The position of the -OH group attached to carbon 1:
in alpha it is below
in beta it is above
What is an isomer?
Molecules having the same chemical formula but a different structural one
e.g. alpha & beta glucose
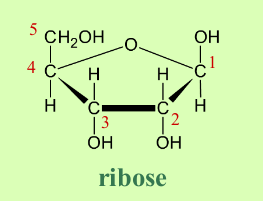
What is the structure of ribose?
What are dissacharides?
formed from 2 monosaccharides
joined by glycosidic bond → condensation reaction
How are disaccharides broken down?
Through a hydrolysis reaction → glycosidic (covalent) bond is broken
Give examples of some dissacharides
Maltose (malt sugar)
Sucrose (table sugar)
Lactose (milk sugar)
How is maltose formed?
2 glucose molecules are joined by an alpha 1-4 glycosidic bond
How is sucrose formed?
Glucose and fructose are joined by an alpha 1-2 glycosidic bond
How is lactose formed?
Galactose and glucose are joined by a beta 1-4 glycosidic bond
How do you test for a reducing sugar?
Using Benedict’s solution:
Cu2+ ions are reduced to Cu+ when solution is boiled → brick red precipitate
sucrose doesn’t react
Why does sucrose not react with Benedict’s soultion?
It is a non-reducing sugar so it doesn’t react
Part of the molecule that needs to react is already in the glycosidic bond
How can sucrose react with Benedict’s solution?
Sucrose needs to be hydrolysed first:
boil sucrose with acid to neutralise it
boil it with Benedict’s
What are polysaccharides?
Polymers containing many monosaccharides & linked together by glycosidic bonds (condensation reaction)
e.g. starch, cellulose & glycogen
What are the two types of starch?
Amylose (α helix) - 20% of starch
Amylopectin (branched starch) - 80%
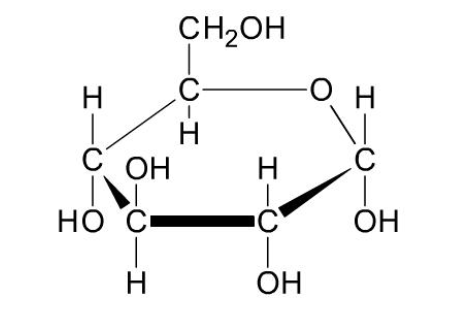
What is the structure of starch?
What is the structure of amylose?
Unbranched helix-shaped chain with 1-4 glycosidic bonds between α-glucose molecules
The helix shape enables it to be more compact and thus it is more resistant to digestion
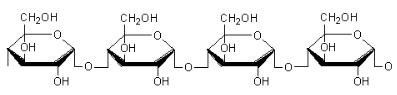
What is the structure of amylopectin?
Highly branched
α glucose molecules joined by α1-4 glycosidic bonds with α1-6 branches every 20-30 monomers
What are the properties of starch?
Compact
Insoluble
Many monomers → longer to digest than glucose
What is the use of starch?
It is a storage molecule for plants
→ They are stored as granules in plastids
What is cellulose?
It is a polysaccharide and the main part of cell walls
What is the structure of cellulose?
long chains of b-glucose molecules joined by b1-4 glycosidic bonds
glucose chains form rope-like microfibrils
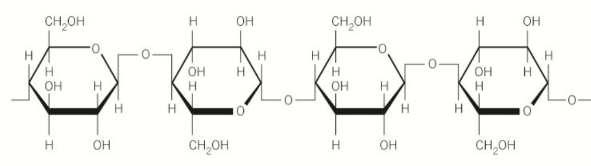
What are the properties of cellulose?
Very strong → provides support
Freely permeable → allows water & solutes to leave cell
What is glycogen?
It is the storage sugar found in animals
What is the structure of glycogen?
Similar structure to amylopectin but more branches
1-4 glycosidic bonds between α-glucose molecules & also 1-6 glycosidic bonds between glucose molecules creating a branched molecule
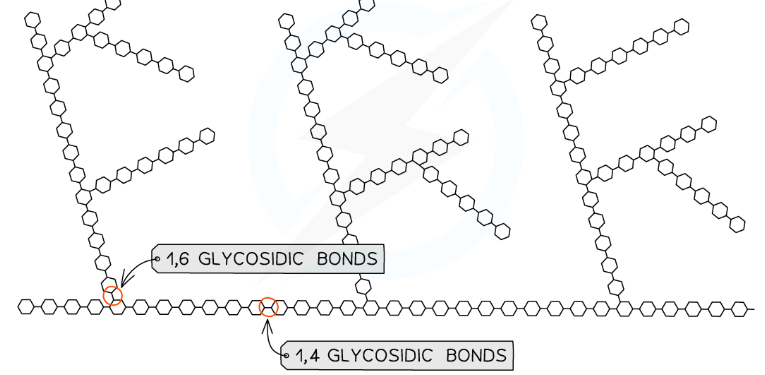
What are the properties of glycogen?
Can quickly be hydrolysed when energy supply needed
Made mostly by livers & muscles
Where is glycogen stored?
Stored as granules in the cytoplasm of cells
What is the structure of triglycerides?
Made up of glycerol & 3 fatty acids
Joined by ester bonds formed in condensation reactions
What is the structure of saturated fats?
Fatty acid chain contains max no. of H atoms
→ no carbon to carbon double bonds & no more hydrogen can be added
Only single bonds between carbons
What is the structure of unsaturated fats?
Have at least one carbon to carbon double bond
What is the structure of phospholipids?
2 fatty acids bonded to glycerol
3rd fatty acid is replaced with a phosphate group
How are triglycerides formed?
Through condensation reactions
Bond is formed between each fatty acid & the glycerol (ester bond)
→ Hydroxyl group (OH) on the fatty acid molecule bonds to a H atom on the glycerol by condensation, releasing a molecule of water
How are triglycerides broken down?
Through hydrolysis reactions (e.g. digestion)
A molecule of water is added to each ester bond to break it apart
Triglyceride splits up into glycerol & 3 fatty acids
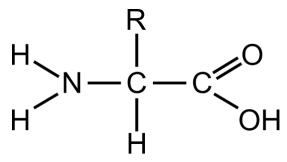
What is the general formula of amino acids?
Contains an amino group (NH2)
Contains carboxylic acid group
Contains an R group
How are dipeptides formed?
A condensation reaction forms a peptide bond, making a new dipeptide molecule
(2 amino acids)
How are polypeptides formed?
When three or more amino acids are joined
Describe the primary structure of proteins
Peptide bonds formed between amino acids to form a polypeptide chain
Describe the secondary structure of proteins
The shape that the chain of amino acids takes - either alpha-helices or beta-pleated sheets
Describe the tertiary structure of proteins
3D shape of a protein
What bonds are present in proteins?
Hydrogen bonds
Hydrophobic interactions
Disulfide bonds
What are hydrogen bonds?
Non-covalent & weak
Involved in all levels of structure
Where are hydrophobic interactions found?
Between non-polar sections of the protein
What are disulfide bonds?
One of the strongest & most important bond in proteins
Occurs between two cysteine amino acids
Describe the quaternary structure of proteins
Exists in proteins containing more than one polypeptide chain
What is the structure of globular proteins?
Spherical shape by tightly folded polypeptide chains
What is the function of globular proteins?
Transport proteins e.g. haemoglobin, myoglobin etc.
Catalyse reactions in enzymes e.g. lipase
Transmit signals in hormones e.g. insulin
What is a conjugated protein?
A globular protein with a non-protein component called a prosthetic group
What is an example of a conjugated protein? Describe its structure and function
Haemoglobin
→ Water soluble, contains 2 alpha & 2 beta polypeptide chains
→ Carries oxygen in the blood & releases it when necessary
What are the properties of fibrous proteins?
Long, rope-like fibres
High tensile strength
Insoluble in water
→ e.g. collagen, keratin, elastin, silk
What is collagen?
The main component of connective tissue e.g. ligaments, tendons & cartilage
What is keratin?
The main component of hard structures e.g. hair, nails, claws & hooves
What are the key inorganic ions involved in biological processes (Cations)?
Calcium ions (Ca2+)
Sodium ions (Na+)
Potassium ions (K+)
Hydrogen ions (H+)
Ammonium ions (NH4+)
What are the key inorganic ions involved in biological processes (Anions)?
Nitrate (NO3 –)
Hydrogencarbonate (HCO3 –)
Chloride (Cl –)
Phosphate (PO4 3–)
Hydroxide (OH–)