Bonding Unit Chem
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
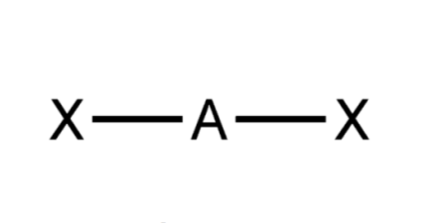
Geometry shape?
Linear
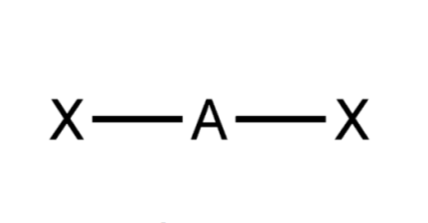
General formula?
AX2
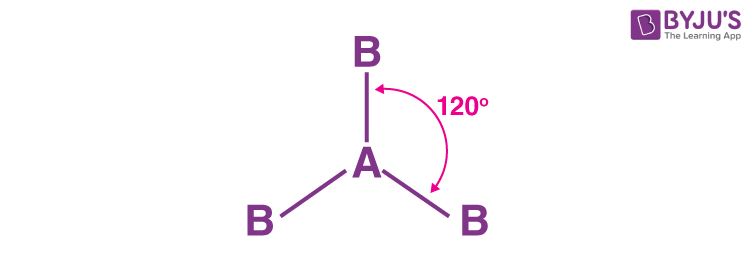
Geometric shape?
trigonal planar
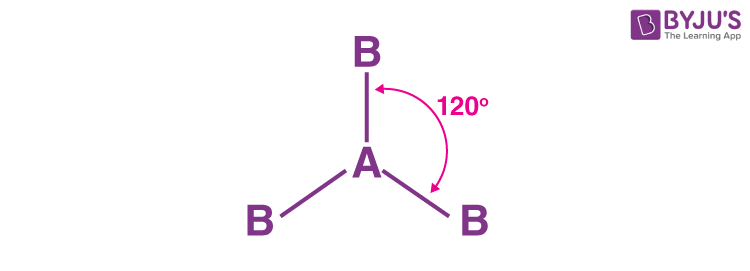
General formula?
AX3
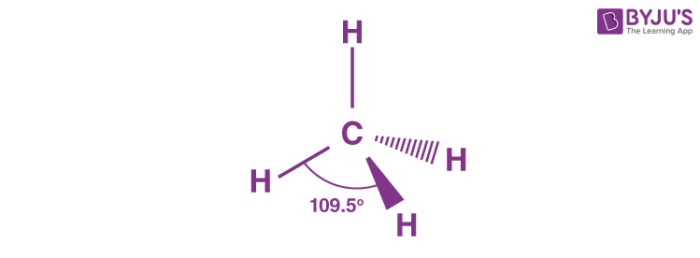
Geometric shape?
Tetrahedral

General formula?
AX4
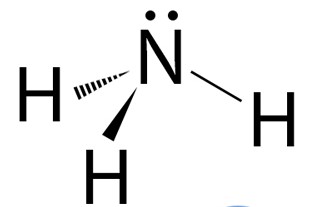
Geometric shape?
Trigonal pyramidal
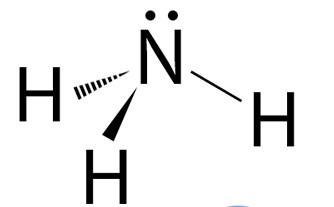
General formula?
AX3E
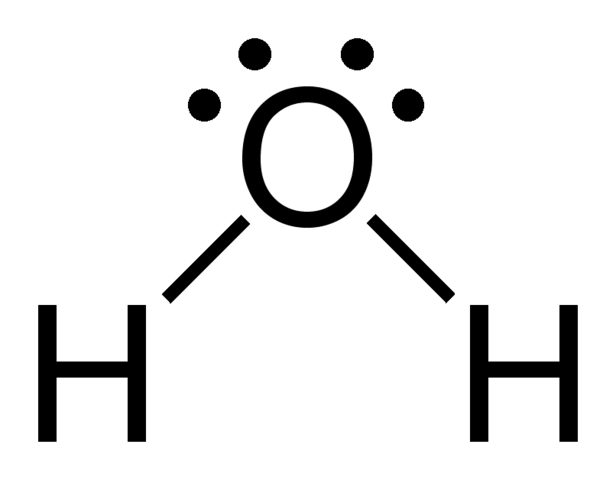
Geometric shape?
Linear bent / angular
General formula?
AX2E2
What is electronegativity?
The ability of an atom to attract electrons in a bond
Where is the highest EN on the periodic table?
up and right
Where is the lowest EN on the periodic table?
down and left
Do noble gases have EM?
No
Do noble gases have any bonding electrons?
No
metal atoms ____ electrons
lose
nonmetal atoms _____ electrons
gain
if EN difference is 1.7 or more the bond formed is likely to be ____
ionic
if the EN difference is less than 1.4 the bond formed is likely to be ______
covalent
If the EN difference is between 1.4 and 1.7, the bond will likely be _____ if one of the atoms has an EN of less than 2
ionic
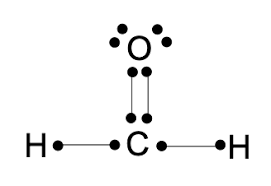
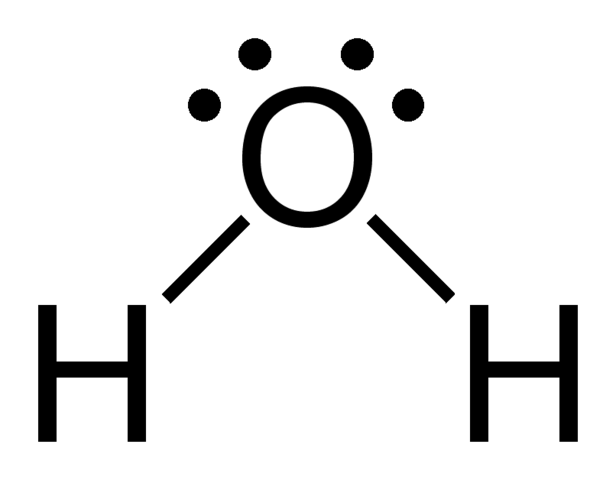
what kind of formula is this?
Lewis
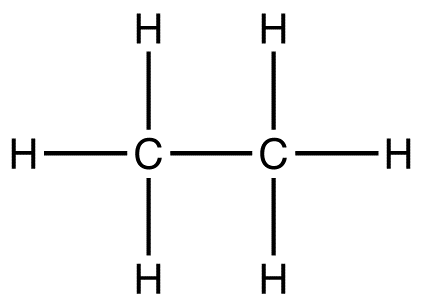
what kind of formula is this?
structural
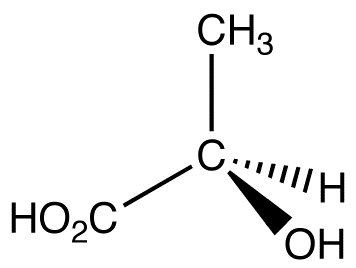
what kind of formula is this?
stereochemical
If the difference in EN is 0.0 - 0.2 the bond is ____
Non polar covalent
If the difference in EN is over 2.0 the bond is ____
ionic
Can polar compounds dissolve in non-polar compounds?
No
What forces determine physical properties?
Intermolecular forces
What intermolecular force does every single molecule/compound have?
London Dispersion Forces
What creates LD?
When electrons in one molecule attract the nuclei (protons) in another
The larger the atom/molecule the _____ the LD force
Larger
What is the weakest intermolecular force?
LD
The more compact/linear (less branches) a molecule is the ______ the LD force
greater
Dipole Dipole forces occur between _________ only
polar molecules
What causes Dipole-Dipole forces?
The positive end of one molecule being attracted to the negative end of a different molecule
What is the strongest intermolecular force?
Hydrogen bonding
Hydrogen bonds only happen between hydrogen and ____ or ____ or _____
NOF (Nitrogen, Oxygen, Florine)
Why do hydrogen bonds only occur with NOF?
They have high electronegativity
What are intramolecular bonds?
covalent and Ionic bonds
Hydrogen bonds are ____ than covalent bonds
weaker
List the 5 kinds of bonds (Intra and Inter) from highest strength to lowest strength
Ionic, Covalent, Hydrogen Bond, Dipole-Dipole, London Dispersion
Are resonance structures the same as isomers?
No
Why do electrons move in resonance?
They move around to make the electron more stable
Name 3 important resonance molecules
NO3 (Nitrate), O3 (Ozone), C6H6 (Benzene)
What does cyclo mean?
The resonance structure will have a ring formation (eg. Benzene)
What is another name for Benzene?
Cyclobenzene or Benzene ring