Lecture 21 - bioenergetics
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
What is the main source of ATP in eukaryotic cells?
The transfer of electrons from NADH and FADH₂ to the mitochondrial electron transport chain.
What is the final electron acceptor in the mitochondrial ETC?
Oxygen (O₂), which is reduced to water (H₂O).
What is oxidative phosphorylation?
The generation of ATP from ADP and Pi using energy from electrons transferred to oxygen; it depends indirectly on oxygen.
Why is it called “oxidative phosphorylation”?
Because electron transfer (oxidation) is coupled to phosphorylation of ADP to ATP.
Where in the mitochondrion does electron transport occur?
In the inner mitochondrial membrane.
What is located in the mitochondrial matrix?
Enzymes for the TCA cycle, pyruvate dehydrogenase, and oxidation of amino acids and fatty acids.
Why is the inner mitochondrial membrane important?
It’s impermeable and houses the protein complexes of the ETC and ATP synthase.
What is the evolutionary origin of mitochondria?
Mitochondria are thought to be derived from aerobic prokaryotes that formed a symbiotic relationship with early eukaryotes.
What genetic features do mitochondria have?
Mitochondria have their own DNA, ribosomes, and tRNAs.
How many major protein complexes are found in the inner mitochondrial membrane?
Five major protein complexes (Complexes I–V)
Which protein complexes make up the mitochondrial electron transport chain (ETC)?
Complexes I through IV
What is the role of Complex V in the mitochondrion?
It synthesizes ATP from ADP and Pi using the proton gradient (ATP synthase)
What is the name of Complex I in the mitochondrial ETC?
NADH-Q oxidoreductase
What is the name of Complex II in the mitochondrial ETC?
Succinate-Q reductase
What is the name of Complex III in the mitochondrial ETC?
Ubiquinone:cytochrome c oxidoreductase
What is the name of Complex IV in the mitochondrial ETC?
Cytochrome c oxidase
What allows Fe-S proteins to function in electron transfer?
Their electronic structure and the reducible iron atoms within the iron-sulfur cofactors enable them to accept and donate electrons.
What enables Fe-S proteins to participate in electron transfer?
The reducible iron atoms and electronic structure of their iron-sulfur clusters.
How is iron coordinated in Fe-S proteins?
Iron atoms are typically coordinated by the sulfur atoms of cysteine side chains (thiol groups) and inorganic sulfide (S²⁻).
Are Fe-S proteins similar to heme groups?
Yes, both contain iron that cycles between oxidation states to transfer electrons, but Fe-S clusters use sulfur (not porphyrin rings) to coordinate the iron.
What is the respirasome?
A supercomplex in the inner mitochondrial membrane composed of ETC Complexes I, III, and IV.
Why is the formation of the respirasome important?
It allows for faster and more controlled electron transfer between complexes.
Does cytochrome c permanently bind to the respirasome?
No, cytochrome c binds the respirasome only during electron transfer.
Which ETC complexes are included in the respirasome?
Complexes I, III, and IV.
What is ubiquinone (Q)?
A lipid-soluble electron carrier in the ETC that accepts 2 electrons from Complex I or II (aka succinate dehydrogenase).
What is the reduced form of ubiquinone?
Ubiquinol (QH₂)
Why is ubiquinone lipid-soluble?
Its long hydrocarbon tail makes it hydrophobic, allowing it to diffuse within the inner mitochondrial membrane.
Which complexes donate electrons to ubiquinone?
Complex I (NADH-Q oxidoreductase) and Complex II (succinate-Q reductase)
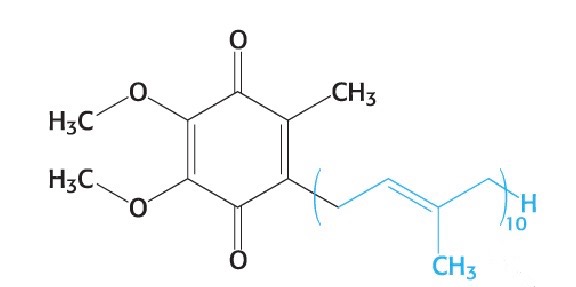
ubiquinone (Q, oxidized)
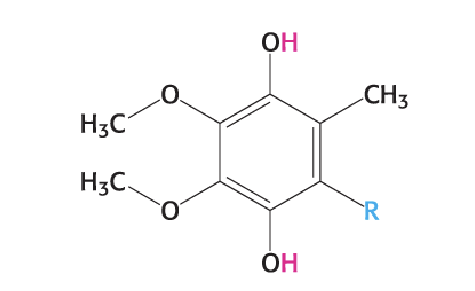
ubiquinol (QH2, reduced)
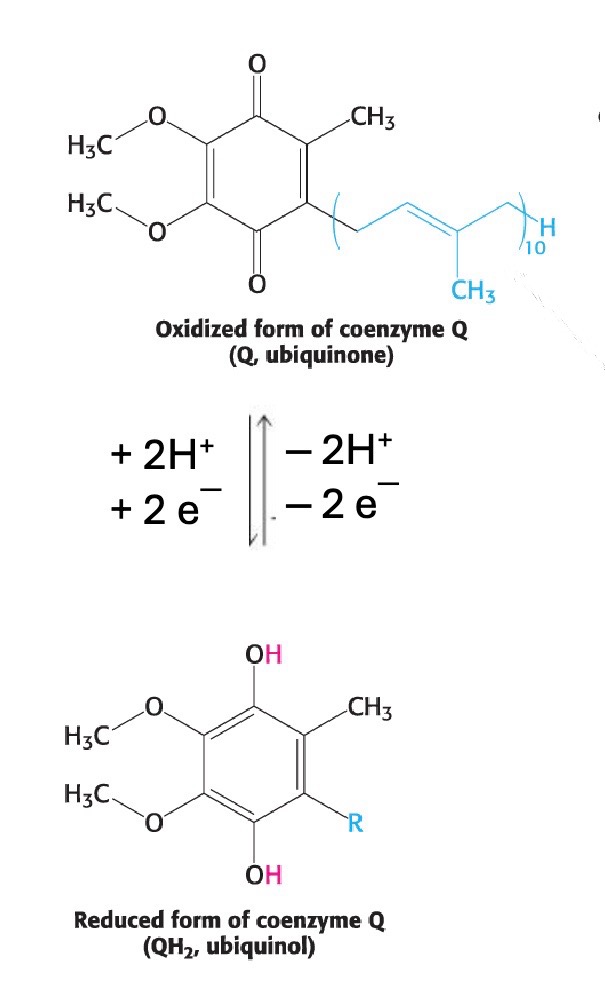
redox of ubiquinone
What is cytochrome c?
A small, water-soluble protein electron carrier with an iron-containing heme group that transfers one electron at a time from Complex III to Complex IV.
How does cytochrome c transfer electrons?
Its iron atom in the heme group cycles between Fe³⁺ (oxidized) and Fe²⁺ (reduced) as it accepts and donates electrons.
What are the electron donor and acceptor for cytochrome c?
Iron in cytochrome c is reduced from Fe³⁺ to Fe²⁺ by Complex III, and oxidized from Fe²⁺ back to Fe³⁺ by Complex IV.
Does the oxidation state of iron in cytochrome c affect its structure?
Yes, changes in iron oxidation state cause subtle conformational changes in cytochrome c.
Why do electrons flow in a specific direction down the electron transport chain?
Because each successive electron carrier has a higher standard reduction potential (greater affinity for electrons) than the one before it.
What is the relationship between standard reduction potential (E°′) and electron flow in the ETC?
Electrons flow from carriers with lower E°′ (like NADH, –0.32 V) to those with higher E°′ (like O₂, +0.82 V), releasing energy.
What is the relationship between ΔG°′ and E°′ in the ETC?
A positive E°′ corresponds to a negative ΔG°′, meaning electron transfer is exergonic and drives ATP synthesis.
What is the overall electron flow pathway in the mitochondrial ETC, and how do NADH and FADH₂ differ in their entry points?
NADH donates electrons to Complex I (NADH-Q oxidoreductase)
→ Electrons pass to ubiquinone (Q)
→ Then to Complex III, cytochrome c, and Complex IV, where O₂ is reduced to H₂O.FADH₂ donates electrons to Complex II (succinate-Q reductase)
→ Electrons also go to ubiquinone (Q), but bypass Complex I.Both pathways converge at ubiquinone, but only NADH uses Complex I, while only FADH₂ uses Complex II.
What is the result of electron transfer from NADH and FADH₂ to oxygen in the ETC?
Electron flow to oxygen drives the pumping of protons from the mitochondrial matrix to the intermembrane space, leading to an accumulation of protons.
This creates a proton gradient (proton-motive force).
Sources of NADH and FADH₂ include glycolysis, the TCA cycle, and fatty acid oxidation.
What is the proton-motive force and how is it generated?
The proton-motive force is the energy stored as a proton gradient across the inner mitochondrial membrane.
It is generated when the free energy from electrons flowing from carriers with low E°′ to high E°′ drives conformational changes in protein complexes, causing proton translocation from the matrix to the intermembrane space.
What are the major respiratory chain complexes in the inner mitochondrial membrane?
Complex I, Complex II, Complex III, and Complex IV.
What is UQ in the mitochondrial electron transport chain?
Ubiquinone, a lipid-soluble electron carrier that shuttles electrons between Complex I/II and Complex III.
How many protons are pumped into the intermembrane space per 2 electrons transferred from NADH to oxygen?
10 protons total:
Complex I: 4 protons
Complex II: 0 protons
Complex III: 4 protons
Complex IV: 2 protons
Why are fewer protons pumped when electrons enter via FADH₂ instead of NADH?
Electrons from FADH₂ enter at Complex II, which does not pump protons, so only 6 protons are translocated in total during electron transfer to oxygen.
How much energy is required to transport 1 mole of protons across the inner mitochondrial membrane?
Approximately 19.4 kJ.
How many protons are pumped out of the mitochondrial matrix per 2 electrons transferred from NADH through the ETC?
10 protons.
How much total energy is required to pump 10 moles of protons across the inner mitochondrial membrane?
About 194 kJ.
What is the ΔG°′ for the transfer of electrons from NADH to molecular oxygen?
Approximately –220 kJ/mol.
Is there enough energy from NADH oxidation to pump protons across the inner mitochondrial membrane?
Yes, the energy released (–220 kJ/mol) exceeds the energy needed (194 kJ) for proton pumping.
Why is proton translocation across the inner mitochondrial membrane a source of potential energy?
Because the inner mitochondrial membrane is impermeable to protons, their translocation creates a charge separation and a proton concentration gradient.
What are the two effects of proton translocation across the inner mitochondrial membrane?
Separation of charge (positive outside, negative inside)
Proton concentration gradient
Together, they generate the proton motive force (pmf).
What is the proton motive force (pmf)?
The combination of the charge separation and proton gradient across the inner mitochondrial membrane that stores potential energy.
What uses the proton motive force to generate ATP?
Complex V (ATP synthase), which uses the pmf to synthesize ATP from ADP and Pi.
What is Complex V also known as?
ATP Synthase
What are the two main components of ATP Synthase?
A membrane-spanning protein channel for proton flow (embedded)
A knob-like peripheral protein with 9 subunits that synthesizes ATP
Where does the peripheral part of ATP Synthase protrude?
Into the mitochondrial matrix
What is the function of the membrane-spanning portion of ATP Synthase?
It allows protons to pass from the intermembrane space back into the matrix
What is the role of the peripheral (matrix-facing) portion of ATP Synthase?
It catalyzes the synthesis of ATP from ADP and Pi
What are the two main parts of FoF₁ ATP Synthase?
Fo: membrane-embedded portion with a rotor made of 8–14 c subunits
F₁: matrix-facing portion with α₃β₃ hexamer and γ, δ, ε subunits
What is the composition of the F₁ (matrix-facing) portion of ATP Synthase?
3 α, 3 β, and one each of γ, δ, ε subunits
How are the α and β subunits arranged in the F₁ portion of ATP Synthase?
As three αβ pairs in a circular hexamer
What connects the c subunits of Fo to the αβ hexamer in F₁?
The δ and ε subunits form a stalk, and the γ subunit runs up the center
How does ATP Synthase use the proton motive force to generate ATP?
Protons flow through channels in the c subunits, causing them (and the attached γ subunit) to rotate. This mechanical rotation within the α₃β₃ hexamer drives ATP synthesis.
What type of energy transformation occurs in ATP Synthase?
Proton motive force (electrochemical) → mechanical rotation → chemical energy (ATP)
What are the three conformations of the β subunit in ATP synthase?
Open (O), Loose (L), and Tight (T)
What occurs in the open conformation of the β subunit?
ADP and Pi enter and bind at the nucleotide-binding site.
What change happens when the γ subunit rotates 120° for the first time?
The β subunit shifts from open to loose conformation, loosely binding ADP and Pi.
What does the loose conformation accomplish?
It holds ADP and Pi in place, preparing them for synthesis.
What happens when the γ subunit rotates another 120°?
The β subunit enters the tight conformation, catalyzing the formation of ATP from ADP and Pi.
What happens during the final 120° rotation of the γ subunit?
The β subunit returns to the open conformation, releasing ATP and allowing a new cycle to begin.
Which subunit of ATP synthase contains the catalytic site for ATP synthesis?
The β subunit of the F₁ portion
How many ATP molecules are produced per full rotation of the γ subunit in ATP synthase?
3 ATP molecules (one per β subunit)
How many ATP molecules can one ATP synthase produce per second under optimal conditions?
30 ATP per second (10 full rotations × 3 ATP)
How many protons are required to generate one ATP molecule?
Approximately 3 protons per ATP
Why is ATP transport necessary after synthesis in the mitochondria?
Most ATP is consumed outside the mitochondria, so ATP must be exported and ADP + Pi imported.
What is the role of ATP-ADP Translocase?
It exchanges mitochondrial ATP for cytosolic ADP across the inner mitochondrial membrane.
What transporter brings Pi into the mitochondrial matrix?
The phosphate carrier, which imports Pi in exchange for OH⁻.
What is the total proton cost to generate and export 1 ATP molecule?
4 protons (3 for ATP synthase rotation, 1 for ADP/Pi/ATP transport)
How many ATP molecules are generated per NADH in the ETC?
2.5 ATP (10 protons ÷ 4 protons per ATP)
How many ATP molecules are generated per FADH₂ in the ETC?
1.5 ATP (6 protons ÷ 4 protons per ATP)
What is the energetic cost of transporting ADP, Pi, and ATP across the inner mitochondrial membrane?
The energy cost is equivalent to pumping one proton out of the matrix.
How many protons are required to generate 1 ATP in the mitochondria?
4 protons total:
3 protons to rotate ATP synthase (Complex V)
1 proton to transport ADP, Pi in and ATP out
What does it mean that ETC electron flow and ATP synthesis are coupled?
It means ATP synthesis via oxidative phosphorylation depends on electron flow through the ETC, and vice versa—if one stops, so does the other.
Why does ATP synthesis stop if electron flow through the ETC stops?
No electron flow → no proton pumping → no proton motive force → ATP synthase cannot rotate → no ATP synthesis.
Give examples of what can stop electron flow through the ETC.
Cyanide (inhibits Complex IV)
Lack of oxygen (no terminal electron acceptor)
What happens if ATP synthesis is disrupted (e.g. by oligomycin)?
The ETC shuts down because proton buildup in the intermembrane space makes proton pumping energetically unfavorable.
What happens when the ETC is uncoupled from ATP synthesis?
Electrons can still flow through the ETC, but no ATP is produced because protons bypass ATP synthase and return to the matrix via alternate paths.
What are 3 things that can disrupt ATP synthesis?
Defective ATP-ADP translocase
Defects in ATP synthase
Inhibitory drugs like oligomycin
What is an uncoupler in oxidative phosphorylation?
An agent that allows protons to return to the mitochondrial matrix without passing through ATP synthase.
What types of uncoupling agents exist?
Some are small lipid-soluble molecules that carry protons; others are protein-based uncouplers.
What is the result of proton return via uncouplers instead of ATP synthase?
The proton motive force is dissipated as heat instead of being used to synthesize ATP—this is called a "proton leak."
What is the function of uncoupling protein 1 (UCP1) in brown adipose tissue (BAT)?
UCP1 creates a channel in the inner mitochondrial membrane that allows protons to bypass ATP synthase, releasing energy as heat.
Where is brown adipose tissue (BAT) found and what is its function?
Found in newborns and small mammals; it generates heat by uncoupling the ETC from ATP synthesis using UCP1.
How does ETC uncoupling generate heat?
Protons leak back into the matrix without making ATP, so their energy is dissipated as heat. About 20% of the proton motive force (pmf) in resting mammals is used this way.
How can ETC uncoupling lead to weight loss?
Less ATP is made per NADH/FADH₂, so more stored fuel (like fatty acids) must be oxidized to meet energy needs, promoting fat loss.
Why were synthetic uncouplers used for weight loss in the 1930s?
They increased energy expenditure by dissipating pmf as heat, causing fat loss—but they were dangerous and sometimes fatal.
Where is brown adipose tissue (BAT) found in adult humans?
Primarily in the neck and upper chest. Thinner people tend to have more BAT.
How do BAT levels correlate with age and body weight?
BAT levels decrease with age and low BAT activity is associated with weight gain.
How might BAT be used therapeutically?
Artificially increasing BAT activity could help prevent weight gain or promote fat loss—though interest has decreased due to GLP-1 inhibitors.