BCEM 393 - Proteins [UNFINISHED]
1/99
Earn XP
Description and Tags
This folder contains flashcard sets based on material from "Biochemistry, A Short Course Fourth Edition" by Tymoczko, Berg, Gatto, and Stryer designed for undergraduate introductory biochemistry. These sets follow University of Calgary BCEM 393 course content
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
100 Terms
Fill in the blanks: in a Fischer projection, every atom is identified and the bonds to the central carbon atom are represented by horizontal and vertical lines. By convention, the horizontal bonds are assumed to project ________, whereas the vertical bonds are assumed to project _______.
Out of the page (toward the viewer), behind the page (away from the viewer
Fill in the blanks: amino acids consist of a _______ bonded to an _______, a ______, a _______, and a _______.
Central carbon, amino group, carboxylic acid group, hydrogen atom, R group
True or False: most amino acids exist in two mirror-image forms.
True
True or False: only D amino acids are constituents of proteins.
False; only L amino acids
True or False: all amino acids have at least two charged groups.
True
Fill in the blanks: at a lower pH, both amino acid functional groups (NH3+ and COO-) are in a _______ form. At a more neutral pH, they are in a _______ form. At a higher pH, they are in a _______ form.
Protonated, zwitterionic, deprotonated
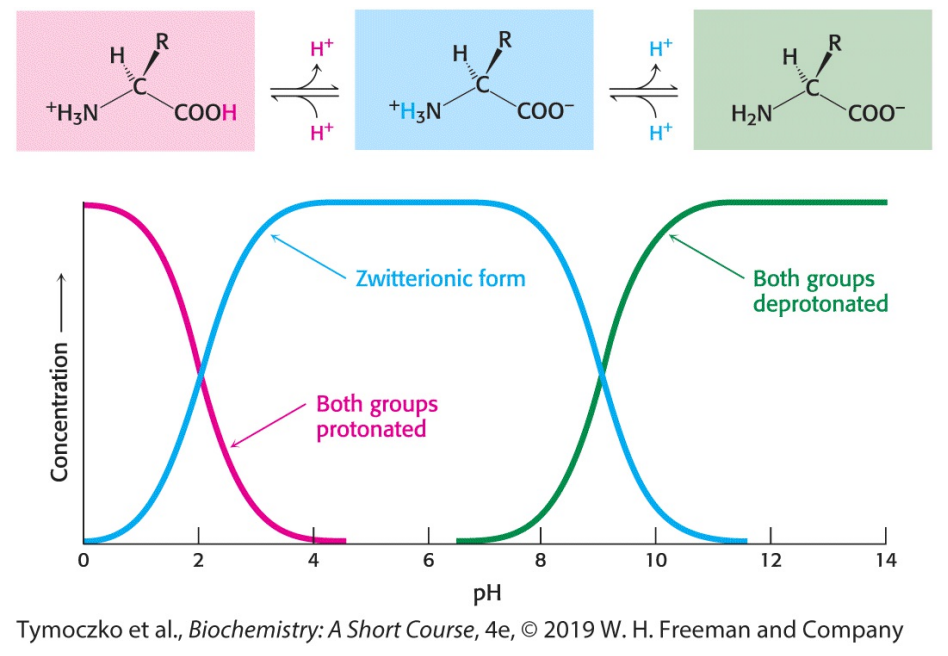
Fill in the blanks: free amino acids in solution at neutral pH exist predominantly as dipolar ions (a.k.a. zwitterions). In the dipolar form, the amino group is ________ and the carboxyl group is _______.
Protonated (NH3+), deprotonated (COO-)
Fill in the blanks: amino acids in acid solution exist where the amino group is ________ and the carboxyl group is _______.
Protonated (NH3+), not dissociated (COOH)
Fill in the blanks: amino acids in basic solution exist where the amino group is ________ and the carboxyl group is _______.
Deprotonated (NH2), deprotonated (COO-)
True or False: under physiological conditions, amino acids exist in the protonated form.
False; dipolar (a.k.a. zwitterionic) form

Identify which depicts the L isomer amino acid and which depicts the D isomer amino acid.
L isomer: left
D isomer: right
Fill in the blanks: seven of the 20 amino acids - _______, _______, _______, _______, ________, _______, and ________ - have readily ionizable side chains. These amino acids are able to form ionic bonds as well as donate or accept protons to facilitate reactions.
Tyrosine, cysteine, arginine, lysine, histidine, aspartic acid, glutamic acid
What is the net charge on the amino acid glycine at pH 7? At pH 12?
pH 7: no net charge (NH3+ and COO-)
pH 12: -1 (NH2 and COO-)
Which amino acids have positively charged R groups at pH 7?
Arginine, lysine, and histidine
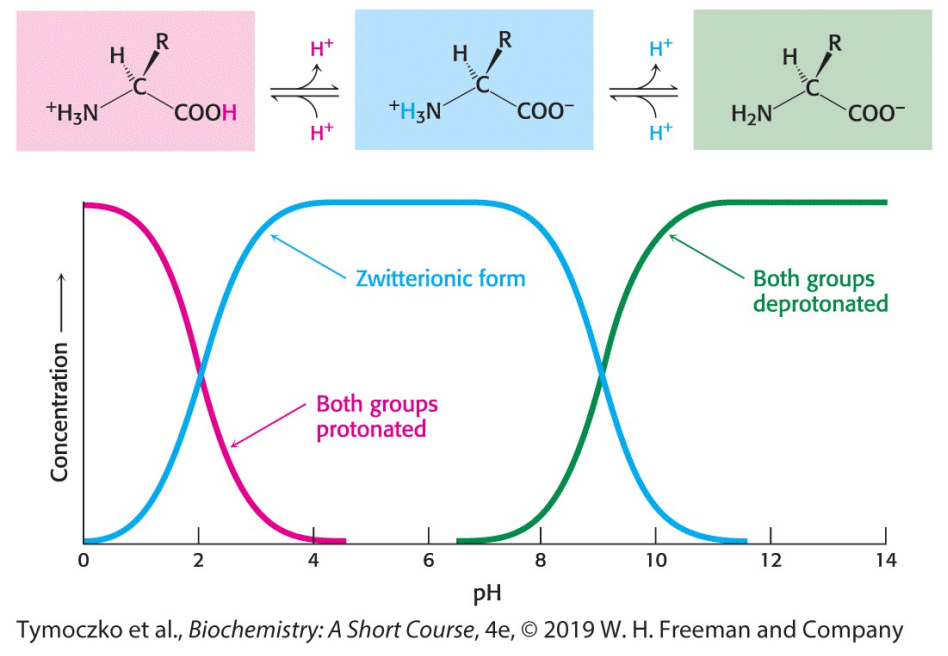
The figure shows the titration curve for a typical amino acid lacking a charged R group. Examine the graph, and determine the pKa for the carboxyl group and the amino group of this amino acid.
Recall that the pKa is the pH at which the concentration of the unionized form of a molecule is equal to the ionized form. The carboxylic acid group has a pKa of slightly more than 2, whereas the amino groups have a pKa of 9
Fill in the blank: the linkage joining amino acids in a protein is called a _______.
Peptide bond (a.k.a., amide bond)
True or False: the formation of a dipeptide from two amino acids is accompanied by the addition of a water molecule.
False; loss of a water molecule
Fill in the blanks: the equilibrium of a peptide bond reaction lies on the side of _______ rather than synthesis under most conditions. Hence, the biosynthesis of peptide bonds requires an input of free energy. Nonetheless, peptide bonds are quite _______ kinetically because the rate of ______ is extremely slow (lifetime of 1000 years in aq solution).
Hydrolysis, stable, hydrolysis
True or False: peptide bonds are quite unstable kinetically because the rate of hydrolysis is relatively fast.
False; peptide bonds are quite stable kinetically because the rate of hydrolysis is extremely slow
Fill in the blank: peptide bonds link ________.
Amino acid residues
Fill in the blanks: a series of amino acids joined by ______ form a _______, and each amino acid unit is called a _______.
Peptide bonds, polypeptide chain, residue
True or False: a polypeptide chain has directionality, sometimes called polarity, because its ends are different.
True
Fill in the blanks: a polypeptide chain has directionality (polarity), because its ends are different: an _______ at one end, and an ________ at the other. By convention, the ________ end is taken to be the beginning of a polypeptide chain, so the sequence of amino acids in a polypeptide chain is written starting with the ________.
α-amino group, α-carboxyl group, amino, amino-terminal residue
True or False: by convention, the carboxyl end is taken to be the beginning of a polypeptide chain rather than the amino end.
False; the amino end is taken to be the beginning
Fill in the blanks: in the pentapeptide Tyr-Gly-Gly-Phe-Leu (YGGFL), tyrosine is the _____-terminal (__-terminal) residue and leucine is the ______-terminal (__-terminal) residue.
Amino, N, carboxyl, C
Fill in the blanks: the amino-terminal is the __-terminal and the carboxyl terminal is the __-terminal.
N, C
Fill in the blanks: a polypeptide chain consists of a regularly repeating part, called the ______, and a variable part, comprising of ________.
Main chain (a.k.a., backbone), distinctive side chains
Fill in the blanks: in a polypeptide chain, the polypeptide backbone is rich in ________. Each residue contains a carbonyl group (C=O), which is a good _______, and, with the exception of proline, an amino group (N-H), which is a good _______.
Hydrogen-bonding potential, hydrogen-bond acceptor, hydrogen-bond donor
Fill in the blanks: in a polypeptide backbone, the carbonyl group (C=O) is a good hydrogen-bond _______, and the amino group (N-H) is a good hydrogen-bond _______.
Acceptor, donor
Fill in the blank: the general term for a polypeptide with a small number of amino acid residues is ________.
Oligopeptide
Fill in the blanks: proteins usually contain between 50 to 2000 amino acid residues. The mean molecular weight of an amino acid residue is about 110 g mol-1, so the molecular weights of most proteins are between _________ g mol-1. We can also refer to the mass of a protein in units of daltons. A protein with a molecular weight of 50,000 g mol-1 has a mass of ________ daltons, or ________ kDa.
5500 and 22,000, 50,000, 50
Fill in the blanks: in some proteins, the linear polypeptide chain is covalently cross-linked. The most common cross-links are ________, formed by the oxidation of a pair of cysteine residues.
Disulfide bonds
Fill in the blanks: disulfide bonds are formed by the ________ of a pair of ________. The resulting unit of two linked ________ is called _________.
Oxidation, cysteine residues, cysteines, cystine
Fill in the blanks: the primary structure of a protein is the linear polymer formed by linking _______ together with _______.
Amino acid residues, peptide bonds
Fill in the blanks: the primary structure of a protein is simply the ________ of the protein, from the ______-terminus to the _______-terminus.
Amino acid sequence, amino, carboxy
True or False: the primary structure determines the three-dimensional structure of a protein, and the three-dimensional structure determines the proteins function.
True
True or False: in primary proteins, the peptide bond is essentially planar.
True
Fill in the blanks: in a primary protein, the peptide bond is essentially planar. Thus, for a pair of amino acids linked by a peptide bond, six atoms lie in the same plane: the _______ atom and ________ group of the first amino acid and the ________ group and the ________ atom of the second amino acid.
α-carbon, CO, NH, α-carbon
Fill in the blanks: in a primary protein, the peptide bond has considerable double-bond character owing to _________: the electrons resonate between a pure _________ and a pure _________.
Resonance structure, single bond, double bond
Fill in the blanks: peptide bonds linking amino acids have resonance structures (pure single bond and pure double bond). This partial double-bond characteristic gives _______ but also prevents ________ about the bond and thus constrains the conformation of the peptide backbone.
Planarity, free rotation
Fill in the blanks: the resonating double-bond character of peptide bonds is expressed in the length of the bond between the _____ and the _____ groups. The ______ distance in a peptide bond (1.32Å) is between the values expected for a single bond (1.45Å) and a double bond (1.27Å) between these atoms.
CO, NH, C-N
True or False: the peptide bond linking amino acids is uncharged, allowing polymers of amino acids linked by peptide bonds to form tightly packed globular structures that would otherwise be inhibited by charge repulsion.
True
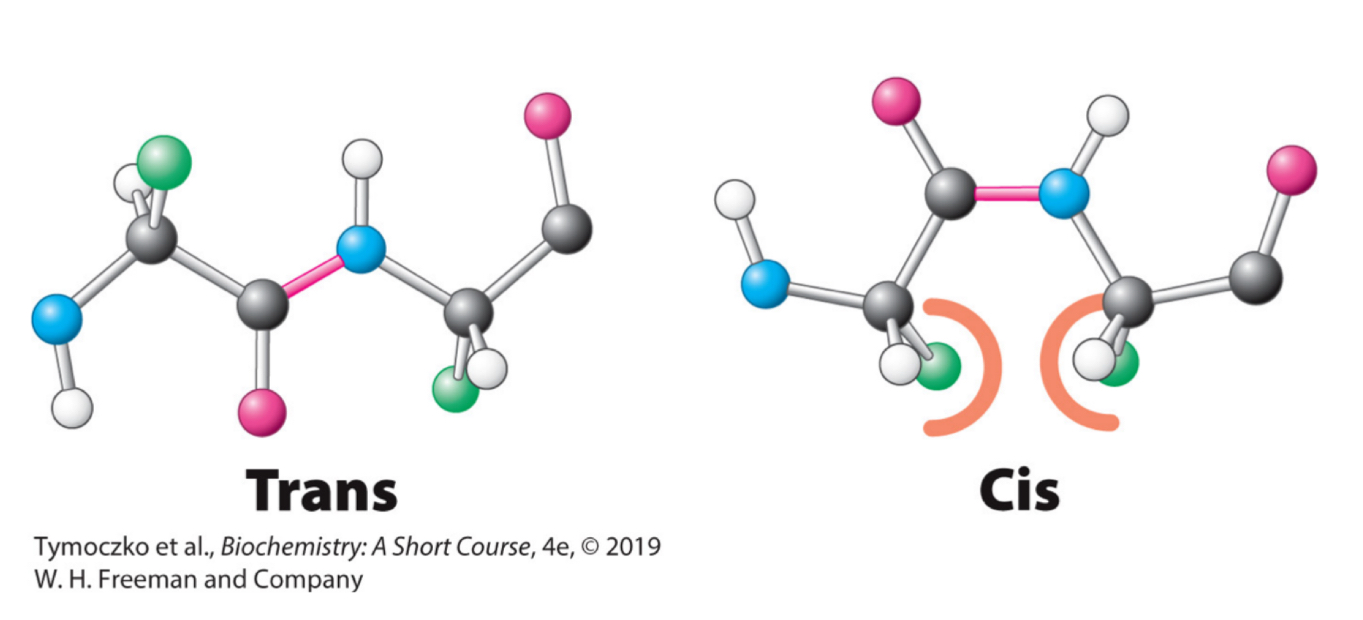
Fill in the blanks: two configuration are possible for a planar peptide bond. In the trans configuration, the two α-carbon atoms are on ________ of the peptide bond. In the cis configuration, these groups are on _________ of the peptide bond. Almost all peptide bonds in proteins are ____.
Opposite sides, the same side, trans
True or False: almost all peptide bonds in proteins are cis.
False; trans
Why are trans conformations of peptide bonds in proteins preferrable over cis conformations?
There are steric clashes between R groups in the cis configuration but not in the trans configuration
Fill in the blank: unlike the peptide bond, the bonds between the amino group and the α-carbon atom and between the α-carbon atom and the carbonyl group are _________.
Pure single bonds
True or False: the bonds between the amino group and the α-carbon atom and between the α-carbon atom and the carbonyl group have resonating conformations (single bond and double bond). Because of this, there is no freedom of rotation.
False; these bonds are pure single bonds, which do allow for freedom of rotation
Fill in the blank: the freedom of rotation about two bonds of each amino acid allows proteins to _________.
Fold in many different ways
Fill in the blank: the angle of rotation about the bond between the nitrogen atom and the α-carbon atom is called ____.
Phi (Φ)
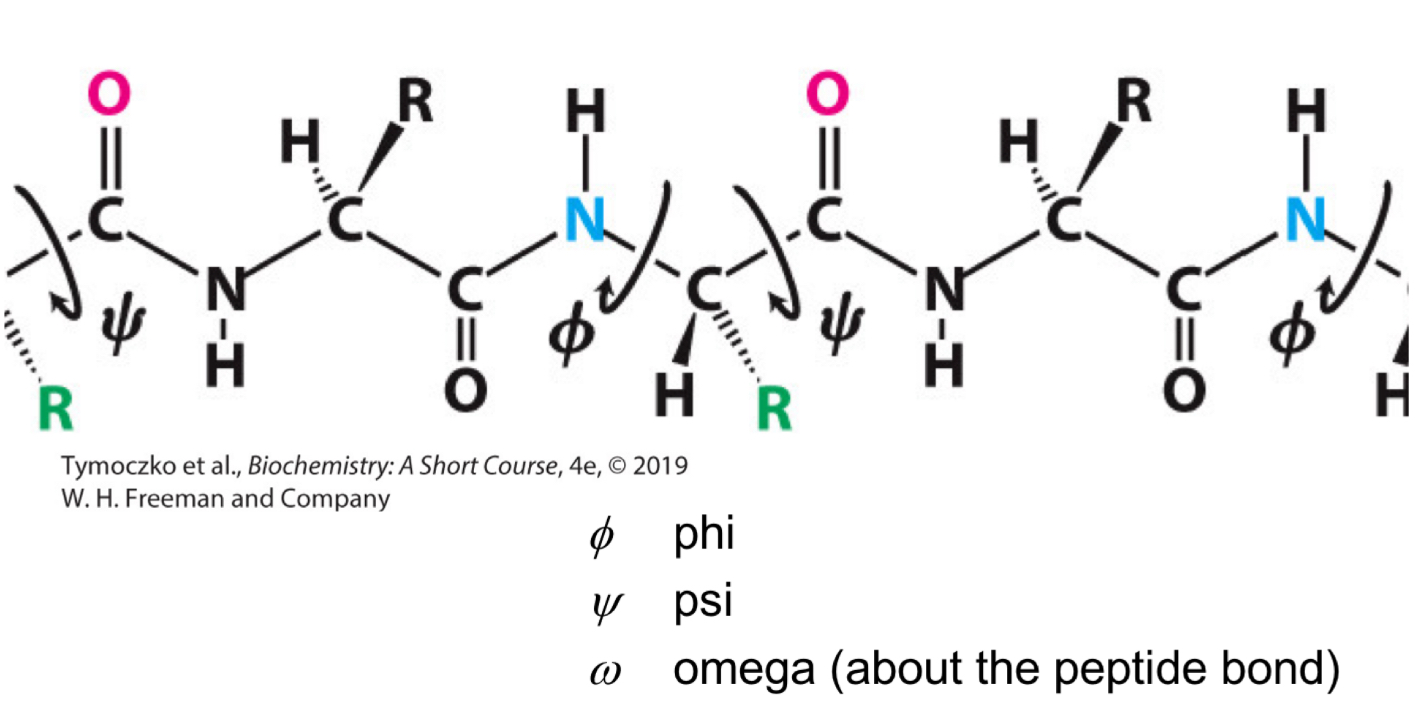
Fill in the blank: the angle of rotation about the bond between the _______ atom and the _______ atom is called phi (Φ).
Nitrogen, α-carbon
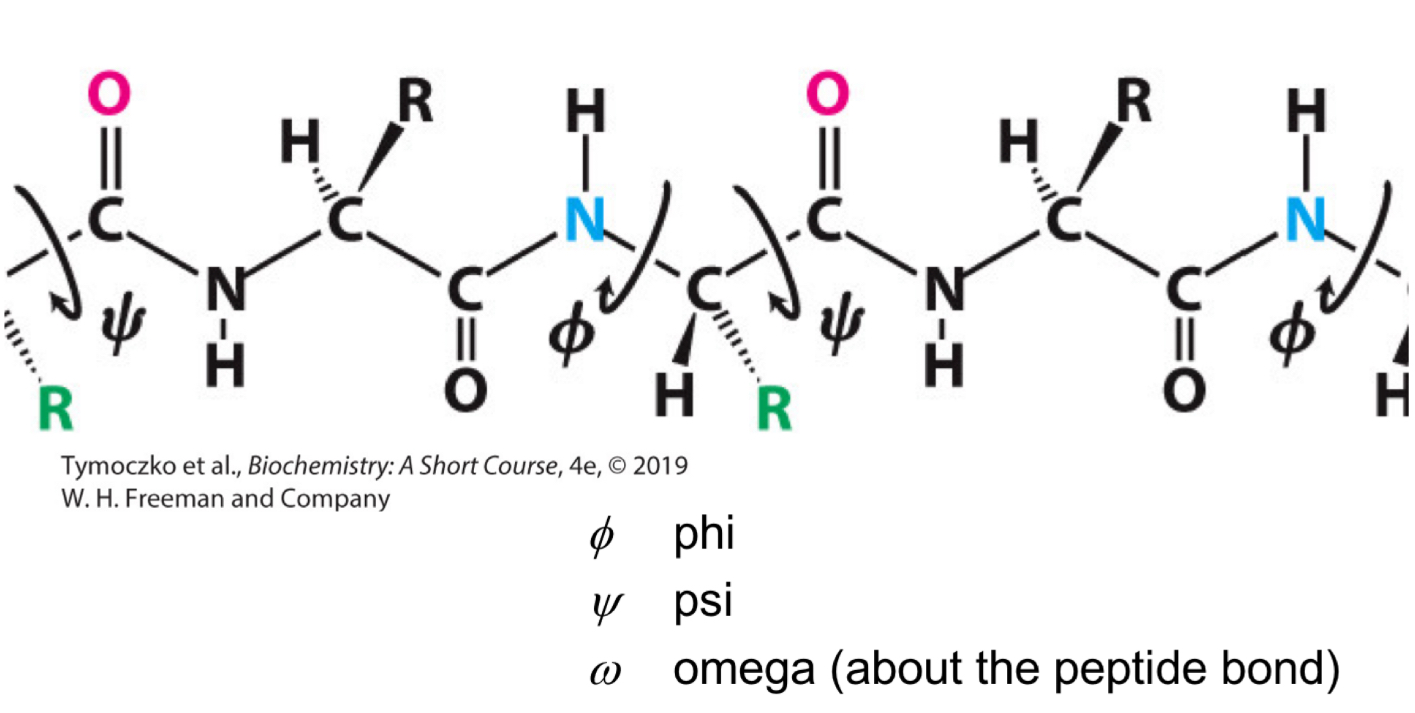
Fill in the blank: the angle of rotation about the bond between the α-carbon atom and the carbonyl carbon atom is called ____.
Psi (Ψ)
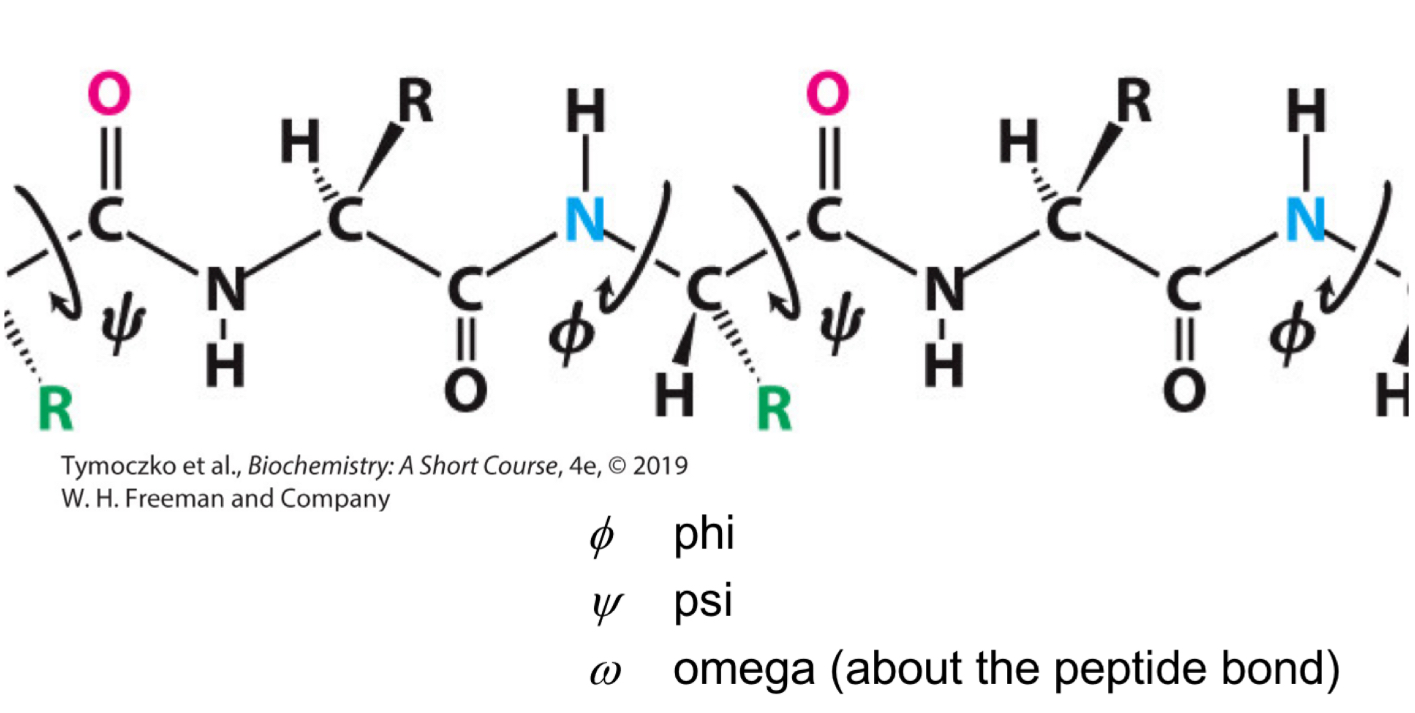
Fill in the blank: the angle of rotation about the bond between the ______ atom and the ______ atom is called psi (Ψ).
α-carbon, carbonyl carbon
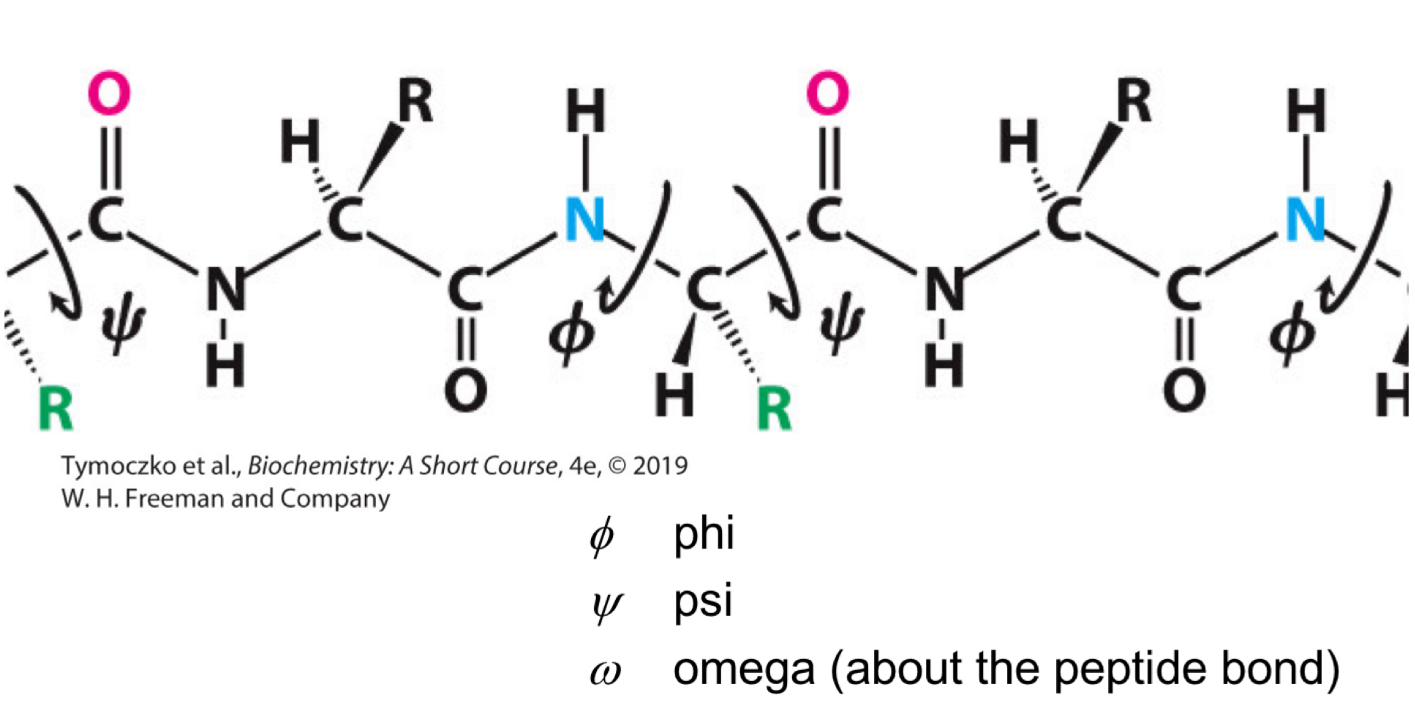
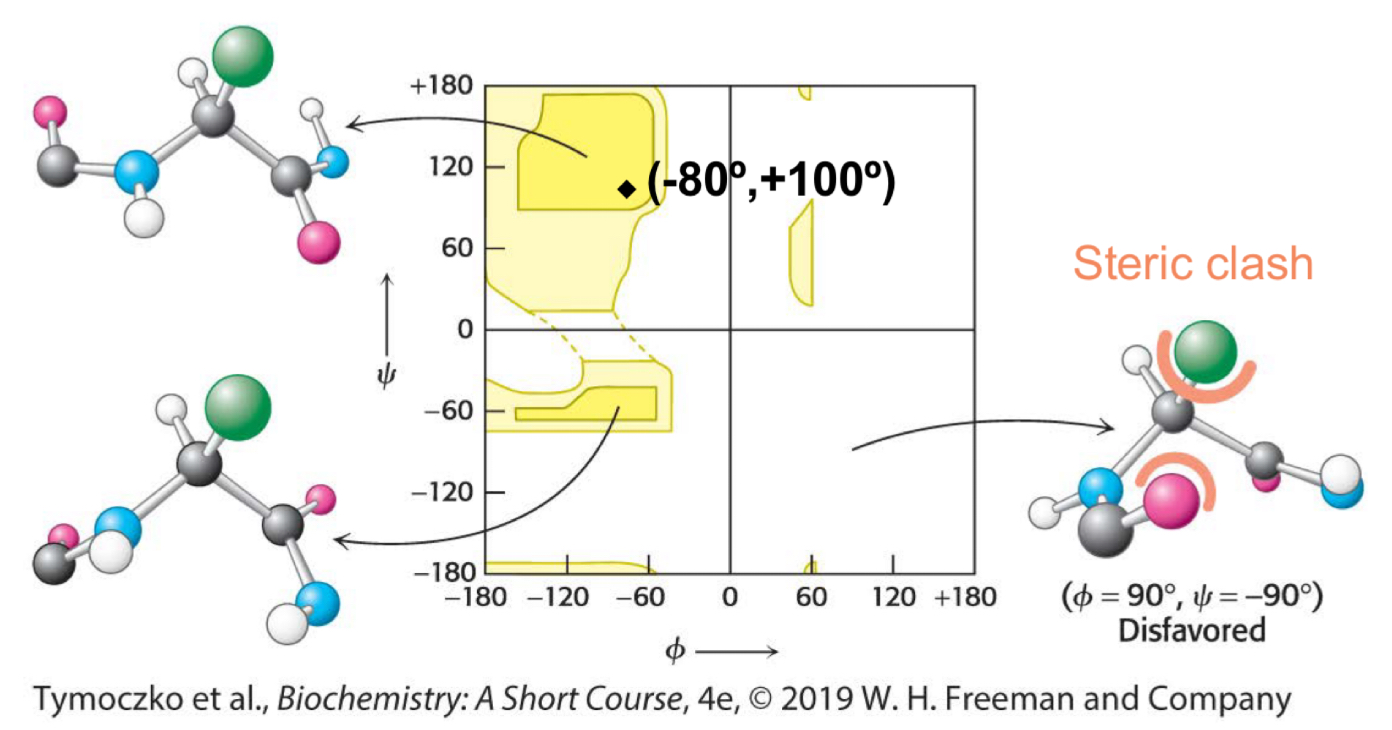
Fill in the blank: a ________ is a diagram of the Φ and Ψ values of possible conformations. Three-quarters of the possible Φ and Ψ combinations are excluded simply by local steric clashes (the fact that two atoms cannot be in the same place at the same time).
Ramachandran plot
Fill in the blanks: in a Ramachandran plot, the top left area aligns with ___________, the top right area aligns with ___________, the bottom left area aligns with __________, and the bottom right area aligns with __________.
Beta sheets, left handed alpha helices, right handed alpha helices, steric clash
Fill in the blanks: alpha helices, beta pleated sheets, and turns are formed by a regular pattern of ________ between the ________ and ________ groups of amino acids that are often near one another in the linear sequence (primary structure).
Hydrogen bonds, peptide NH, CO
Fill in the blanks: the alpha helix is stabilized by ________ between the ___ and ___ groups of the main chain.
Hydrogen bonds, NH, CO
True or False: except for amino acids near the ends of an alpha helix, all of the main-chain CO and NH groups are hydrogen-bonded.
True
Fill in the blanks: there are 3.6 amino acid residues per turn of alpha helix. Thus, amino acids spaced three or four apart in the sequence are spatially ________ to one another in the alpha helix. In contrast, amino acids spaced two apart in the sequence are spatially ________ to one another in the alpha helix. Because of this, amino acids spaced ______ apart are unlikely to make contact.
Quite close, on opposite sides, two
Fill in the blanks: the pitch of the alpha helix is the length of one complete turn along the helix axis and is equal to the product of the translation, ____, and the number of residues per turn, ____, which is ____.
1.5Å, 3.6, 5.4Å
Fill in the blanks: the screw sense of the helix can be right-handed (clockwise) or left-handed (counterclockwise). ________ helices are energetically more favourable because there are fewer steric clashes between the side chains and the backbone. Because of this, essentially all alpha helices found in proteins are ________.
Right-handed, right-handed
True or False: essentially all alpha helices found in proteins are left-handed.
False; right-handed
True or False: all amino acids can be readily accommodated in an alpha helix.
False; not all amino acids can, branching at the β-carbon tends to destabilize alpha helices because of steric clashes
Fill in the blanks: the distance between adjacent amino acids along a beta strand is approximately ___Å, in contrast with a distance of ___Å along an alpha helix.
3.5, 1.5
Fill in the blanks: a beta sheet is formed by linking two or more _________ lying next to one another through _________.
Beta strands, hydrogen bonds
Fill in the blanks: adjacent chains in a beta sheet can run in opposite directions (_______ beta sheet) or in the same direction (________ beta sheet).
Antiparallel, parallel
Fill in the blanks: unlike in alpha helices, in two beta strands that lie next to each other, the last amino acid of one strand and the first amino acid of the adjacent strand are ________ in the amino acid sequence.
Not necessarily neighbors
Fill in the blanks: antiparallel beta strand run in opposite directions. ________ between ___ and __ groups connect each amino acid to _______ amino acid(s) on an adjacent strand, stabilizing the structure. Parallel beta strands run in the same direction. ________ connect each amino acid on one strand with _________ amino acid(s) on the adjacent strand.
Hydrogen bonds, NH, CO, a single, hydrogen bonds, two different
True or False: beta sheets can only be parallel or antiparallel.
False; they can be parallel, antiparallel, or mixed (both parallel and antiparallel)
Fill in the blank: in schematic representations, beta strand are usually depicted by broad arrows pointing in the direction of the __________ end to indicate the type of beta sheet formed (parallel or antiparallel).
Carboxyl-terminal
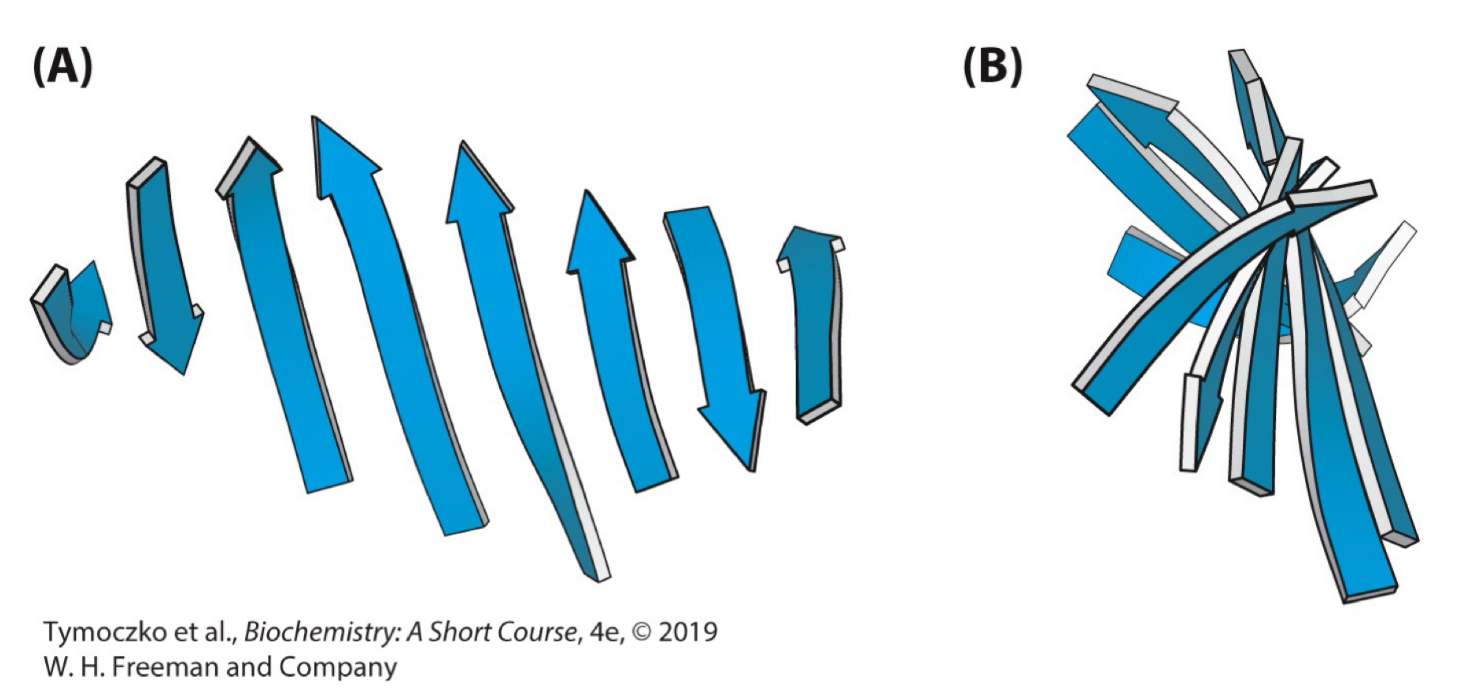
True or False: beta sheets can be almost flat, but most adopt a somewhat twisted shape.
True
Fill in the blanks: proteins often have compact, globular structures. This means that the polypeptide chain has to change direction, often using ________ and _________.
Reverse turns, loops
True or False: loops exposed to an aqueous environment are usually composed of amino acids with hydrophilic R groups.
True
Fill in the blanks: primary structure is the _________, secondary structure is the _________, and tertiary structure is the _________.
Sequence of amino acids, simple repeating structures formed by hydrogen bonds between H and O atoms of the peptide backbone, spatial arrangement of amino acid residues that are far apart in the sequence and to the pattern of disulfide bonds
Fill in the blank: certain combinations of secondary structure are present in many proteins and frequently exhibit similar functions. These combinations are called ________.
Supersecondary motifs
Fill in the blank: some polypeptide chains fold into two or more compact regions that may be connected by a flexible segment of polypeptide chain, like pearls on a string. These compact globular units are called _________.
Domains
True or False: different proteins may have domains in common even if their overall tertiary structures are different.
True
Fill in the blanks: many proteins consist of more than one polypeptide chain in their functional states. Each polypeptide chain in such a protein is called a ________. The quaternary structure describes the arrangement of ________ and the nature of their interactions.
Subunit, subunits
True or False: the polypeptide chains (subunits) of a quaternary structure must be the same.
False; they do not have to be the same
True or False: quaternary structure can be as simple as two identical subunits or as complex as dozens of different polypeptide chains.
True
Fill in the blank: more than one type of subunit can be present in a quaternary structure. For example, human hemoglobin consists of two subunits of one type (designated α) and two subunits of another type (designated β). Thus, the hemoglobin molecule exists as an __________.
α2β2 tetramer (note the α and β have nothing to do with helices or strands in this case, just used for historical reasons)
True or False: the amino acid sequence of a protein determines its three dimensional structure.
True
Fill in the blanks: the classic work of _________ in the 1950s on the enzyme _________ revealed the relation between the amino acid sequence of a protein and its conformation.
Christian Anfinsen, ribonuclease
Explain Christian Anfinsen’s experiment in the 1950s that examined how the elaborate three-dimensional structure of proteins is attained.
Destroy the three-dimensional structure of a protein (added a chaotropic agent to disrupt non-covalent bonds, urea, to a solution of protein, ribonuclease, then cleave the disulfide bonds reversibly with β-mercaptoethanol)
Determine the conditions to restore its tertiary structure (after previous step, protein was denatured. When denatured ribonuclease was freed of β-mercaptoethanol and urea by dialysis, it slowly regained activity)
This showed that the information needed to specify the three-dimensional structure of protein is contained in its amino acid sequence (the native structure is the most thermodynamically stable structure - sequence specifies conformation)
Explain Levinthal’s Paradox.
If each residue of a 100-residue protein can assume three different conformations, the total number of structures would be 3100 (5 × 1047)
If the conversion of one structure into another were to take 10-13 seconds, the total search time would be 5 × 1047 × 10-13s (5 × 1034s, or 1.6 × 1027 years)
It would take way too long for even a small protein to fold properly by randomly trying out all possible conformations
Since proteins can fold in seconds, they cannot be sampling all possible conformations
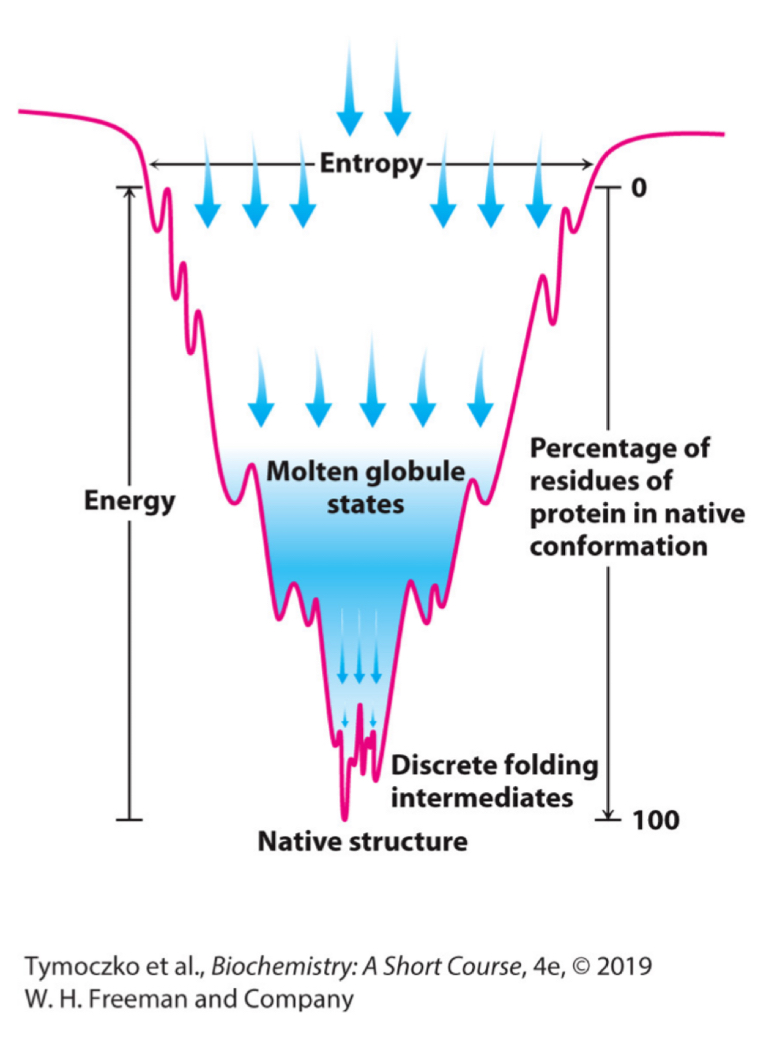
Explain the concept of the folding funnel for the folding of proteins.
The width of the funnel represents all possible conformations of the unfolded protein
The depth of the funnel represents the energy difference between the unfolded and the native protein
Each point on the surface represents a possible three-dimensional structure and its energy value
The funnel suggests that there are alternative pathways to the most stable structure
For the structure Gly-His what is the charge on the peptide at pH 4.0? at pH 7.5?
pH 4.0: +1
pH 7.5: 0
Write the following sequence using one-letting abbreviations: Thr-Glu-Pro-Ile-Val-Ala-Pro-Met-Glu-Tyr-Gly-Lys.
TEPIVAPMEYGK
Estimate the net charge of the following sequence at pH 7 and at pH 12: Thr-Glu-Pro-Ile-Val-Ala-Pro-Met-Glu-Tyr-Gly-Lys.
pH 7: -1
pH 12: -4
Which of the following amino acids would likely disrupt an alpha helix:
a. Tryptophan
b. Phenylalanine
c. Glycine
d. Proline
d.
List some of the differences between an alpha helix and a beta strand.
The helix is a condensed, coiled structure, with the R groups bristling outward from the axis of the helix. The distance between two adjacent amino acids is 1.5 Å. The strand is a fully extended polypeptide chain, and the side chains of adjacent amino acids point in opposite directions. The distance between adjacent amino acids is 4.5 Å. Both structures are stabilized by hydrogen bonding between components of the polypeptide backbone
Which of the following amino acids are likely to be found on the interior of a protein:
a. Valine
b. Aspartic acid
c. Leucine
d. Lysine
a. and c.
What are the levels of protein structure? Describe the type of bonds characteristic of each level.
Primary structure: peptide bond
Secondary structure: local hydrogen bonds between components of the polypeptide backbone
Tertiary structure: various types of noncovalent bonds between R groups that are far apart in the primary structure
Quaternary structure: various noncovalent bonds between R groups on the surface of subunits
A mutation that changes an alanine residue in the interior of a protein into valine is found to lead to a loss of activity. However, activity is regained when a second mutation at a different position changes an isoleucine residue into glycine. How might this second mutation lead to a restoration of activity?
The first mutation destroys activity because valine occupies more space than alanine does, so the protein must take a different shape, assuming that this residue lies in the closely packed interior. The second mutation restores activity because of a compensatory reduction of volume; glycine is smaller than isoleucine
How would treating with heat contribute to protein denaturation?
Heat would increase the thermal energy of the chain. The weak bonds holding the chain in its correct three-dimensional structure would not be able to withstand the wiggling of the backbone, and the tertiary structure would be lost. Often, the denatured chains would interact with each other, forming large complexes that precipitate out of solution
How would treating with a hydrophobic detergent contribute to protein denaturation?
Detergents would denature the protein by essentially turning it inside out. The hydrophobic residues in the interior of the protein would interact with the detergent, whereas the hydrophilic residues would interact with one another and not with the environment
How would treating with large changes in pH contribute to protein denaturation?
All ionic interaction, including hydrogen bonds, would be disrupted, resulting in protein denaturation
Glycine is a highly conserved amino acid residue in the evolution of proteins. Why?
Glycine has the smallest side chain of any amino acid. Its size is often critical in allowing polypeptide chains to make ight turns or to approach one another closely
Most proteins have hydrophilic exteriors and hydrophobic interiors. Would you expect this structure to apply to proteins embedded in the hydrophobic interior of a membrane? Explain.
Some proteins that span biological membranes are “the 2675 exceptions that prove the rule” because they have the reverse distribution of hydrophobic and hydrophilic amino acids. For example, consider porins, proteins found in the outer membranes of many bacteria. Membranes are built largely of hydrophobic chains. Thus, porins are covered on the outside largely with hydrophobic residues that interact with the neighboring hydrophobic chains. In contrast, the center of the protein contains many charged and polar amino acids that surround a water-filled channel running through the middle of the protein. Thus, because porins function in hydrophobic environments, they are “inside out” relative to proteins that function in aqueous solution
Proteins that span biological membranes often contain alpha helices. Given that the insides of membranes are highly hydrophobic, predict what type of amino acids will be in such a helix. Why is an alpha helix particularly suitable for existence in the hydrophobic environment of the interior of a membrane?
The amino acids will be hydrophobic in nature. An alpha helix is especially suitable for crossing a membrane because all of the amide hydrogen atoms and carbonyl oxygen atoms of the peptide backbone take part in intrachain hydrogen bonds, thus stabilizing these polar atoms in a hydrophobic environment
The alpha and beta subunits of hemoglobin bear a remarkable structural similarity to myoglobin. However, in the subunits of hemoglobin, residues that are hydrophilic in myoglobin are hydrophobic. Why?
Recall that hemoglobin exists as a tetramer, whereas myoglobin is a monomer. Consequently, the hydrophobic residues on the surface of hemoglobin subunits probably take part in van der Waals interactions with similar regions of the other subunits and are shielded from the aqueous environment by these interactions