Naming Compounds-Medical Science
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
What does Alkane mean? What is the ending of the compound name if the molecule is alkane?
Connections between carbon and hydrogen is only single bonds. The ending is -ane
What does Alkene mean? What is the ending of the compound name if the molecule is alkene?
Connections between carbon and hydrogen has at least one double bond. Ending is -ene
What does Alkyne mean? What is the ending of the molecule name if the molecule is alkyne?
Connections between carbon and hydrogen has at least one triple bond. Ending is -yne
What does the prefix mean?
How many carbons
Meth- means how many carbons?
One
Eth- means how many carbons?
Two
Pro- means how many carbons?
Three
But- means how many carbons?
Four
Pent- means how many carbons?
Five
Hex- means how many carbons?
Six
Hept- means how many carbons?
Seven
Oct- means how many carbons?
Eight
Non- means how many carbons?
Nine
Dec- means how many carbons?
Ten
What is the structure of aliphatic hydrocarbons? What are examples of it?
The structure is usually a straight chain with examples being alkanes, alkenes, and alkynes.
What is the structure of aromatic hydrocarbons? What is an example?
The structure is a closed or cyclic ring with a benzene ring composted of single/double bonds being an example.
In a cis or trans isomer, the bonds between carbon atoms must be ___ or ___, with the 4 elements around carbon not all ____.
double or triple, not all hydrogen(two must be a different elements)
In a cis or trans isomer, what determines the structure to be cis/trans?
The positioning of the two different elements/compounds attached to the carbons.
Describe a Cis isomer
The two compounds(not hydrogen) are on the same side. The compound is usually polar because of unequal sharing of electrons.
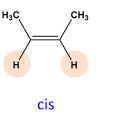
Describe a Trans isomer
The two compounds(not hydrogen) are on opposite sides. The compound is usually nonpolar because equally sharing of electrons.
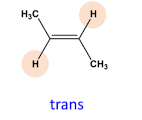
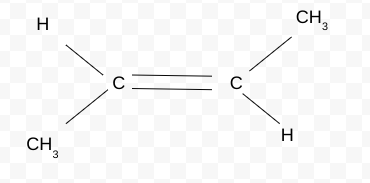
Name the Cis/trans isomer(Z/E)
trans-but-2-ene
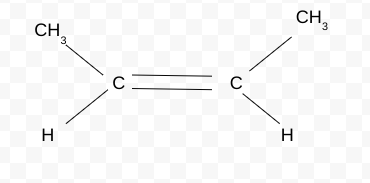
Name the Cis/trans isomer(Z/E)
cis-but-2-ene
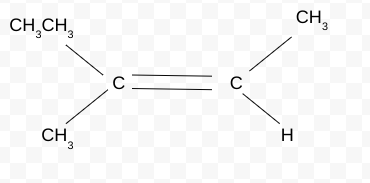
Name the Cis/Trans isomer(Z/E)
Z-3-methylpent-2-ene
priority groups are both on same side→ Cis but Z because the groups are different
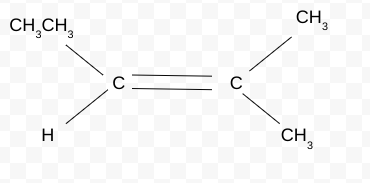
Naming the Cis/Trans isomer(Z/E)
Trick Question!!!! There must be 2 different elements attached to C
2-methylbut-2-ene
What is Cahn-Ingold-Prelog?
Finds which group gets priority(to find main structure) by comparing atomic mass and electronegativity.
For example, CH3CH3 has a greater mass than CH3(on the left) because it has more carbon and CH3 has a greater mass than H(on the right).
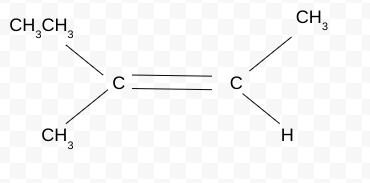
What is a substituent? Give examples
Any element/compound that replaces hydrogen
Examples: side chain,halogen,functional group
What are the two types of carbon compounds?
1) Straight chain(main road)
2) Branch chain(side roads)
The main chain has/doesn’t more carbons then the branch.
Has
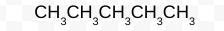
Is this chemical structure, molecular formula, or stick diagram?
Molecular Formula
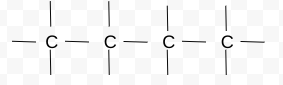
Is this chemical structure, molecular formula, or stick diagram?
Chemical Structure
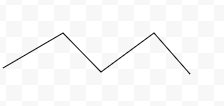
Is this chemical structure, molecular formula, or stick diagram?
Stick diagram
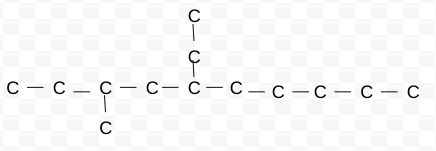
Name this branch chain
remember these steps:
1) name main road
2)how many carbons in each branch
3)what # carbon is branch on→use lowest H
4)combine to name
5-ethyl,3-methyldecane
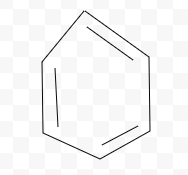
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Aromatic
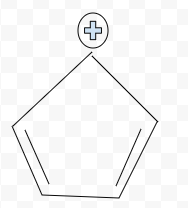
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Anti-Aromatic
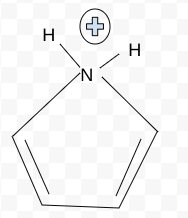
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
NonAromatic
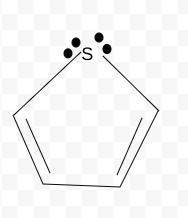
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Aromatic
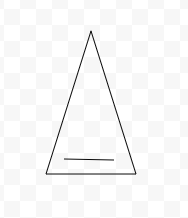
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Non Aromatic
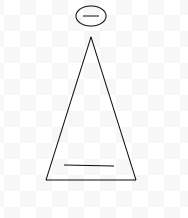
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Antiaromatic
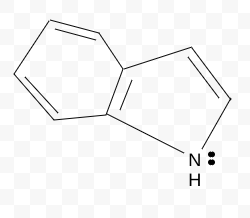
Aromatic, Antiaromatic, or NonAromatic
Notes:
Aromatic→cyclic(closed ring structure), every molecule (carbon) must have p-orbital(besides Sp3), conjugated(pi electrons(p-orbitals) must overlap with adjacent p-orbital), planar(flat, 2-D structure), follows Huckel’s rule (4n+2)
Antiaromatic→cyclic, every molecule(carbon) must have p-orbital), conjugated, planar
Nonaromatic→non cyclic(linear molecule), Sp,Sp2,Sp3 carbons, not conjugated, non planar(3-D shape), add # of pi electrons
Aromatic
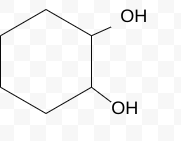
Name the molecule
1,2-cyclohexanediol
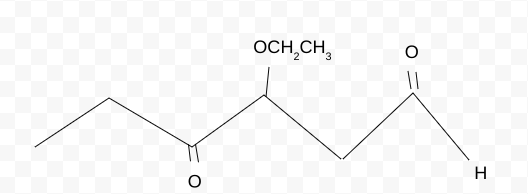
Name the molecule
3,4-ethoxyoxyhexanal
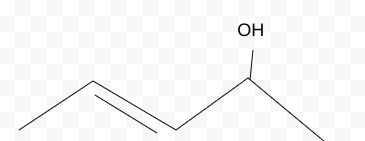
Name the molecule
3-pentene-2-ol
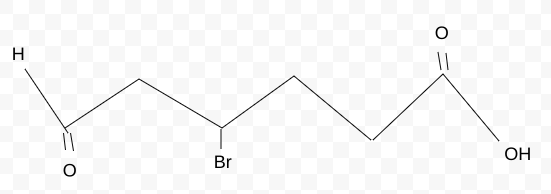
Name the molecule
4,6-bromooxohexanic acid
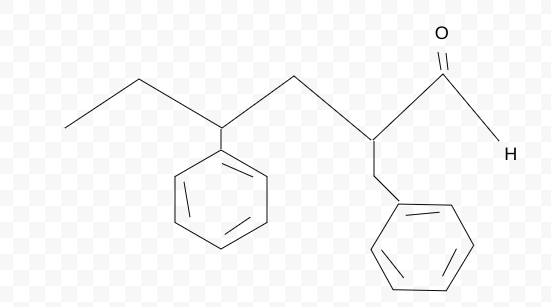
Name the molecule
Note:
Benzyl:directly attached to the functional group
Phenyl:not directly attached to functional group
2,4-benzylphenylhexanal
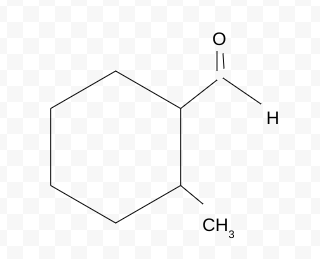
Name the molecule
2-methylcydohexanecarbaldehyde
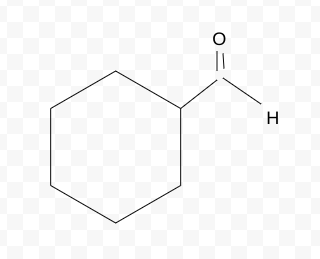
Name the molecule
cyclohexanecarbaldehyde
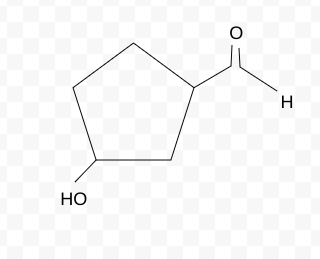
Name the molecule
3-hydroxycyclopentanecarbaldehyde