microbrial metabolism
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
All Cells Have Two Main Goals ?
The sum of all chemical reactions in the cell =………
All Cells Have Two Main Goals
Synthesize new parts
Build cell walls, membranes, ribosomes, and nucleic acids.
Requires anabolic reactions (energy input).
Harvest energy to power reactions
Break down nutrients to release energy.
Involves catabolic reactions (energy release).
The sum of all chemical reactions in the cell = metabolism.
2 branches of metabolism
how are they interconnected
Catabolism
Anabolism
These two processes are interconnected — energy from catabolism fuels anabolism.
what is catabolism
exer or endo?
Energy is captured as …..
Catabolism
Breaks down large molecules → smaller ones.
Releases energy (exergonic).
This energy is captured as ATP or in electron carriers (NADH, FADH₂).
Example: Glycolysis, Krebs Cycle, Electron Transport Chain.
what is anabolism
eder or exergonic
Uses …. produced by catabolism.
Anabolism
Builds large macromolecules from smaller subunits.
Requires energy (endergonic).
Uses ATP produced by catabolism.
Example: synthesis of proteins, nucleic acids, or lipids.
Energy Principles
Energy ?
Potential energy?
Kinetic energy?
1st Law of Thermodynamics?
Energy Principles
Energy = capacity to do work.
Potential energy: stored (chemical bonds, water behind a dam).
Kinetic energy: movement (flowing water).
1st Law of Thermodynamics: Energy can’t be created or destroyed, only converted.
type of energy in microbes
Photoautotrophs?
Chemoorganotrophs:?
2nd Law of Thermodynamics?
Photoautotrophs: Use sunlight → convert kinetic light energy to chemical energy.
Chemoorganotrophs: Use organic compounds → depend on photosynthetic energy indirectly.
2nd Law of Thermodynamics: Energy conversions are inefficient; heat is lost.
Free Energy (ΔG)
Exergonic reaction?
Endergonic reaction?
Energy coupling?
Free Energy (ΔG)
Exergonic reaction (ΔG < 0): Energy is released — spontaneous.
Endergonic reaction (ΔG > 0): Energy is required — non-spontaneous.
Energy coupling: Exergonic reactions (like glucose breakdown) power endergonic ones (like ATP synthesis).
what are enzymes ?
Enzymes are biological catalysts that speed up reactions without being consumed.
Ho do enzymes work ?
Bind specific substrates at the active site.
Form an enzyme-substrate complex → converts to product.
Lower activation energy needed for reaction.
Cofactors?
Coenzymes?
Cofactors: Inorganic ions (Mg²⁺, Zn²⁺, Fe²⁺).
Coenzymes: Organic molecules, often derived from B vitamins (e.g., NAD⁺, FAD, NADP⁺).
types of enzymes
Exoenzymes?
Endoenzymes?
Constitutive enzymes?
Regulated enzymes?
Exoenzymes: Work outside the cell (e.g., amylase, cellulase).
Endoenzymes: Stay inside the cell (most enzymes are like this).
Constitutive enzymes: Always produced (e.g., glucose metabolism enzymes).
Regulated enzymes: Turned on/off depending on substrate availability.
Enzyme Stability and Inhibition Environmental Factors:
Each enzyme has an optimal………and …….concentration.
Each enzyme has an optimal temperature, pH, and salt concentration.
Inhibitors
Competitive inhibitors:
Noncompetitive inhibitors:
Inhibitors:
Competitive inhibitors:
Bind the active site.
Block substrate binding.
Example: Sulfa drugs.
Noncompetitive inhibitors:
Bind elsewhere (allosteric site).
Change enzyme shape.
Example: mercury poisoning (irreversible).
Allosteric Regulation
Allosteric Regulation
Enzyme’s activity is controlled by a molecule binding at a site other than the active site.
Causes feedback inhibition — the final product of a pathway can inhibit the first enzyme to prevent overproduction.
ATP – The Energy Currency
ATP =……….
Composed of …….+ ……. +………
ATP → ……+ ……. releases energy.
…… + ……… → ATP stores energy.
ATP – The Energy Currency
ATP = Adenosine Triphosphate.
Composed of adenine + ribose + 3 phosphate groups.
ATP → ADP + Pi releases energy.
ADP + Pi → ATP stores energy.
Ways to Make ATP
Substrate-level phosphorylation?
Oxidative phosphorylation?
Photophosphorylation?
Substrate-level phosphorylation: Direct transfer of phosphate during a reaction.
Oxidative phosphorylation: Uses proton motive force from electron transport.
Photophosphorylation: Uses sunlight to generate ATP.
Redox Reactions
Oxidation?
Reduction?
Electron carriers like…………shuttle electrons in metabolism.
Oxidation: Loss of electrons or hydrogen (energy released).
Reduction: Gain of electrons or hydrogen (energy stored).
Electron carriers like NAD⁺/NADH, FAD/FADH₂ shuttle electrons in metabolism.
Central Metabolic Pathways
Glycolysis?
TCA Cycle (Krebs Cycle)?
Electron Transport Chain (ETC)?
Glycolysis:
Splits glucose (6C) → 2 pyruvate (3C).
Produces ATP and NADH.
TCA Cycle (Krebs Cycle):
Completes oxidation of pyruvate → CO₂.
Produces ATP, NADH, FADH₂.
Electron Transport Chain (ETC):
Uses NADH/FADH₂ to create a proton gradient.
Drives ATP synthesis (oxidative phosphorylation).

what does this illustrate
llustrates cellular respiration:
Glucose oxidation → CO₂ + H₂O + ATP.
Electron carriers (NADH, FADH₂) transport energy.
Proton Motive Force (PMF) across the membrane powers ATP synthase → Oxidative Phosphorylation.
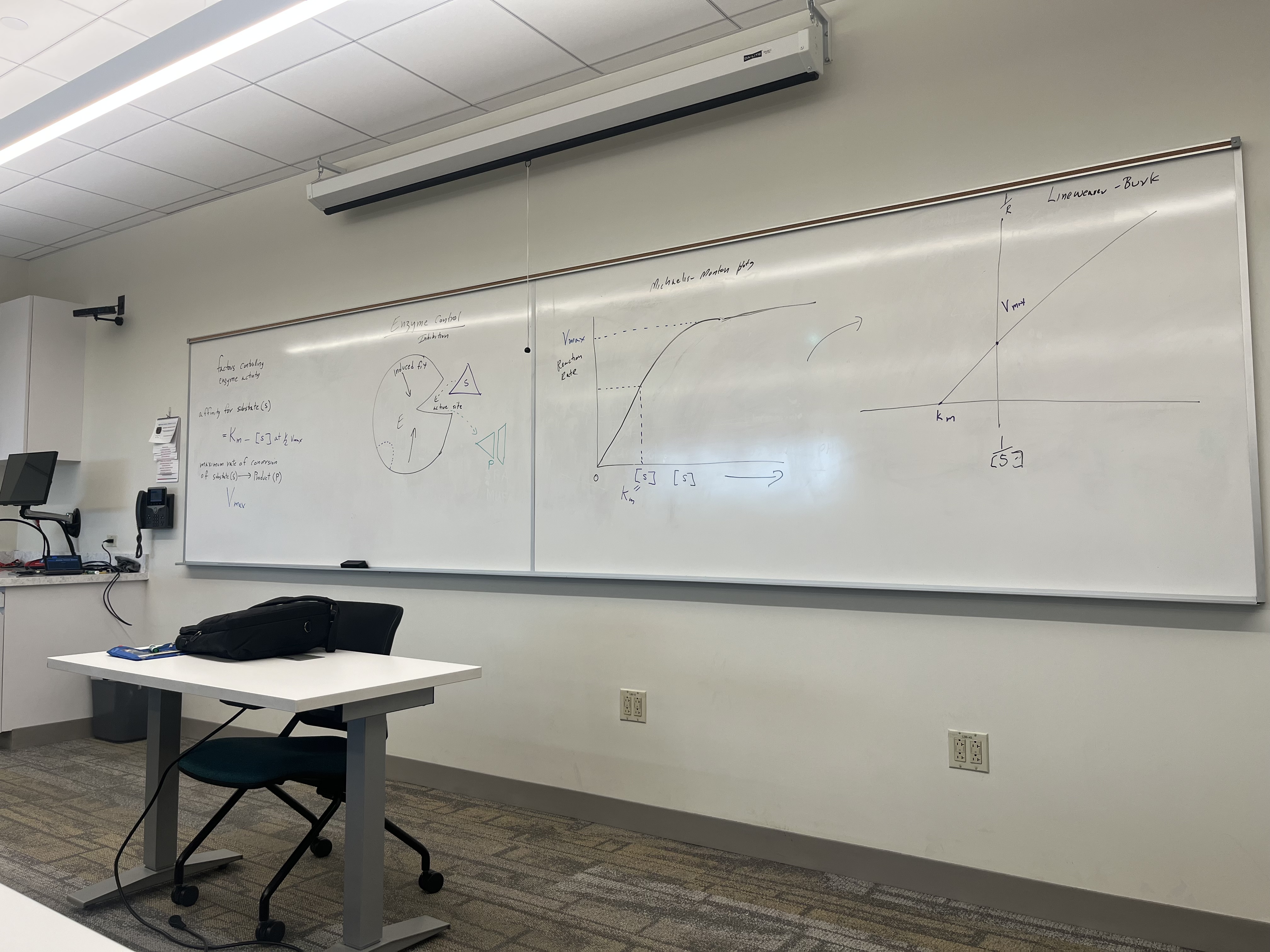
What does the graph show?
Graphs show enzyme kinetics:
Michaelis-Menten curve: Shows how reaction rate depends on substrate concentration.
Lineweaver-Burk plot: Double-reciprocal graph used to calculate Km (affinity) and Vmax (maximum rate).
Catabolism is all about …….. to release energy — primarily from …...
Catabolism is all about breaking molecules down to release energy primarily from glucose.
Respiration → oxidative phosphorylation
Respiration transfers electrons from glucose → electron transport chain (ETC).
The ETC uses those electrons to generate a proton motive force (PMF) — an energy gradient across a membrane.
The PMF powers ATP synthase to make ATP by oxidative phosphorylation.
Aerobic Respiration
…..is the ……. at the end of the ETC.
Produces the ……(in prokaryotes, up to 38 ATP per glucose).
Aerobic Respiration
O₂ is the terminal electron acceptor at the end of the ETC.
Produces the most ATP (in prokaryotes, up to 38 ATP per glucose).
Anaerobic Respiration
A molecule other than …. (e.g.,….) serves as the……..
Uses a …….
Produces (more or less?) ATP than aerobic respiration because the alternate acceptors have …...
Common in …..only
A molecule other than oxygen (e.g., nitrate, sulfate, or carbonate) serves as the terminal electron acceptor.
Uses a modified TCA cycle.
Produces less ATP than aerobic respiration because the alternate acceptors have lower electron affinity.
Common in prokaryotes only (many bacteria do this).
what is The Central Metabolic Pathways
These are the main routes that glucose follows as it’s broken down for energy and carbon skeletons.
Glycolysis
Start:
End:
Energy Summary
Net yield:
Phases
Investment phase:
Pay-off phase:
Glycolysis
Start: 1 molecule of glucose (6 carbons)
End: 2 molecules of pyruvate (3 carbons each)
Energy Summary
Net yield:
2 ATP (via substrate-level phosphorylation)
2 NADH (reduced electron carriers)
Phases
Investment phase:
2 ATP are used to add phosphate groups to glucose.
Glucose splits into two 3-carbon molecules.
Pay-off phase:
Each 3-carbon molecule is oxidized to pyruvate.
Produces 4 ATP total (2 net gain).
Produces 2 NADH.
Transition Step (Happens ….per glucose)
Converts …. → 2……
Key events:
Each pyruvate loses ….. → forming a ……...
Electrons are transferred to …. → ….
The ……. group attaches to ……., forming …….., which enters……
Transition Step (Happens twice per glucose)
Converts 2 pyruvate → 2 acetyl-CoA.
Key events:
Each pyruvate loses one CO₂ → forming a 2-carbon acetate.
Electrons are transferred to NAD⁺ → NADH.
The 2-carbon acetyl group attaches to Coenzyme A, forming acetyl-CoA, which enters the TCA cycle.
Tricarboxylic Acid (TCA) Cycle / Krebs Cycle
Occurs ….. per glucose (once for each ….).
What happens:
Completes oxidation…..
Electrons are transferred to….
Per Glucose:
? released
? produced
? NADH
? FADH₂
Generates…….
Tricarboxylic Acid (TCA) Cycle / Krebs Cycle
Occurs twice per glucose (once for each acetyl-CoA).
What happens:
Completes oxidation of glucose → all 6 carbons now released as CO₂.
Electrons are transferred to NAD⁺ and FAD to form NADH and FADH₂.
Per Glucose:
4 CO₂ released
2 ATP produced
6 NADH
2 FADH₂
Generates precursor metabolites (used in biosynthesis)
Respiration and the Electron Transport Chain (ETC)
All the NADH and FADH₂ made in glycolysis, transition, and TCA cycle carry electrons to the…….
Respiration and the Electron Transport Chain (ETC)
All the NADH and FADH₂ made in glycolysis, transition, and TCA cycle carry electrons to the ETC.
Chemiosmotic Principle
Proposed by?
it explains what?
Steps?
Chemiosmotic Principle
Proposed by Peter Mitchell (1961) — it explains how ATP is made during respiration.
Steps:
ETC passes electrons through a series of membrane-bound carriers.
As electrons move, protons (H⁺) are pumped across the membrane → creates proton motive force (PMF).
The PMF drives ATP synthase, which spins like a turbine to make ATP from ADP + Pi.
The final electron acceptor (O₂ or another molecule) receives the electrons.
Location of the ETC
Prokaryotes:?
Eukaryotes:?
Location of the ETC
Prokaryotes: cytoplasmic membrane
Eukaryotes: inner mitochondrial membrane
Energy Use of PMF
The proton motive force isn’t just for ATP. Prokaryotes also use it to?
Energy Use of PMF
The proton motive force isn’t just for ATP. Prokaryotes also use it to:
Power flagella rotation (movement)
Drive active transport of molecules into the cell
Generate ATP via ATP synthase
ETC in Prokaryotes
Highly …….
E. coli is an excellent example:
Aerobic respiration?
Anaerobic respiration?
Anaerobic respiration?
ETC in Prokaryotes
Highly variable — different bacteria use different combinations of electron carriers.
E. coli is an excellent example:
Aerobic respiration: uses O₂ as terminal acceptor.
Anaerobic respiration: uses nitrate or other inorganic compounds.
Anaerobic respiration yields less ATP because alternate acceptors have lower electron affinities (less energy released per electron).
ATP Yield in Prokaryotic Aerobic Respiration
In eukaryotes, yield is usually …. due to…..
Source | Process | ATP Produced |
|---|---|---|
Glycolysis | Substrate-level | 2 |
TCA Cycle | Substrate-level | 2 |
Subtotal (Substrate-level) | 4 ATP | |
Glycolysis (NADH) | Oxidative phosphorylation | 6 |
Transition step (NADH) | Oxidative phosphorylation | 6 |
TCA cycle (NADH + FADH₂) | Oxidative phosphorylation | 22 |
Subtotal (Oxidative) | 34 ATP | |
Total (Theoretical max) | 38 ATP |
In eukaryotes, yield is usually 36 ATP due to transport losses in mitochondria.
If the cell can’t use respiration it must …..
🔹 Fermentation Pathway?
If the cell can’t use respiration (no ETC or no suitable electron acceptor), it must regenerate NAD⁺ another way — or glycolysis will stop.
🔹 Fermentation Pathway
Pyruvate (or a derivative) serves as the terminal electron acceptor.
This regenerates NAD⁺ from NADH so glycolysis can continue.
Only 2 ATP per glucose (from glycolysis).
No TCA or ETC involved.
Fermentation produces varied end products depending on the organism:
Fermentation produces varied end products depending on the organism:
End Product | Example Organism | Use |
|---|---|---|
Ethanol | Yeast | Alcoholic beverages |
Lactic acid | Lactobacillus | Yogurt, cheese |
Butyric acid | Clostridium | Solvent production |
Propionic acid | Propionibacterium | Swiss cheese flavor |
2,3-Butanediol | Enterobacter | Diagnostic test |
Mixed acids | E. coli | Indicator for ID tests |
Catabolism of Organic Compounds Other than Glucose
Microbes can extract …. from many sources — not just glucose.
Catabolism of Organic Compounds Other than Glucose
Microbes can extract energy from many sources — not just glucose.
Polysaccharides & Disaccharides
Amylases:?
Cellulases: ?
Disaccharides?
🔹 Lipids
Lipases: split fats into?
🔹 Proteins
Proteases:?
Amino group is?
Carbon skeletons?
Polysaccharides & Disaccharides
Amylases: break down starch
Cellulases: break down cellulose
Disaccharides (e.g., lactose) are hydrolyzed to monosaccharides, then enter glycolysis.
🔹 Lipids
Lipases: split fats into glycerol + fatty acids
Glycerol → converted to dihydroxyacetone phosphate (DHAP) → enters glycolysis.
Fatty acids → degraded via β-oxidation → acetyl-CoA → TCA cycle.
🔹 Proteins
Proteases: break proteins into amino acids.
Amino group is removed (deamination).
Carbon skeletons enter central metabolism (glycolysis or TCA).
Anabolic Pathways – Building from Precursor Molecules
Energy and intermediates from catabolism are now used to……
These subunits all come from ……. generated during ……., …….., and ……….
Anabolic Pathways – Building from Precursor Molecules
Energy and intermediates from catabolism are now used to build macromolecules:
Macromolecule | Subunits | Source Pathway |
|---|---|---|
Carbohydrates | Sugars | Glycolysis intermediates |
Proteins | Amino acids | Glycolysis, pyruvate, TCA intermediates |
Lipids (Fats) | Fatty acids + glycerol | Acetyl-CoA and glycolysis intermediates |
Nucleic acids | Nucleotides | Glycolysis & TCA cycle intermediates |
Energy Flow
Glucose → ATP
If oxygen (or another acceptor) is available → …….
If not → ………
Energy Flow
Glucose → Glycolysis → Pyruvate → Acetyl-CoA → TCA Cycle → ETC → ATP
If oxygen (or another acceptor) is available → respiration
If not → fermentation