Kaplan MCAT General Chemistry Chapter 8: The Gas Phase Edited
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
matter can exist in three physical forms called phases or states
solid liquid gas
like liquids, gases are classified as
fluids
because they
because they can flow and take on the shapes of their containers.
however, they differ from liquids because they
move rapidly and are far apart from each other
what exists between gas molecules and what does this cause
very weak intermolecular forces which allows them to easily expand to fill any volume and to be very easily compressible
what variables do we use to define the gaseous state
PVTn
SI unit for gas pressure and conversion
1 atm= 760 mmHg= 760 torr= 101.325 kPa (pascal)
units of pressure relationships
1 atm= 760 mmHg= 760 torr= 101.325 kPa
sphygmomanometers
an instrument used to measure blood pressure (mmHg)
sphygmo-: relating to the pulse or pulsation.
-manometers: an instrument for measuring the pressure acting on a column of fluid, especially one with a U-shaped tube of liquid in which a difference in the pressures acting in the two arms of the tube causes the liquid to reach different heights in the two arms.
describe how a mercury barometer works
Atmospheric pressure creates a downward force on the pool of mercury at the base of the barometer
the mercury in the column exerts an opposing force (its weight) based on its density.
The weight of the mercury creates a vacuum in the top of the tube.
When the external air exerts a higher force than the weight of the mercury in the column, the column rises.
When the external air exerts a lower force than the weight of the mercury, the column falls.
Thus, a reading can be obtained by measuring the height of the mercury column (in mm), which will be directly proportional to the atmospheric pressure being applied.
is atmospheric pressure the only thing that can exert this force
no
For instance, a clinical blood pressure cuff creates a force that is opposed by the person’s systolic and diastolic arterial blood pressure.
processes involving gas take place under
STP
STP
273 K (0 celcius) and 1 atm
what to note about STP
not the same as standard state conditions
STP is for gas law calculations, the other is for standard enthalpy, entropy, free energy changes, and electrochemical cell voltage
standard state conditions are: 298k, 1 ATM, 1M concentration
ideal gas
no intermolecular forces and occupies no volume
When do real gases deviate from ideal behavior?
Under high pressure, low volume and temperature conditions.
Combined gas law
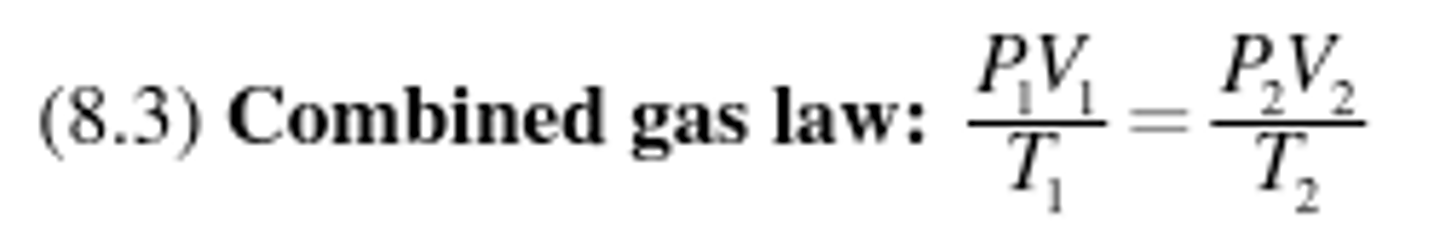
Avogrado's principle eqtn

Boyle's law
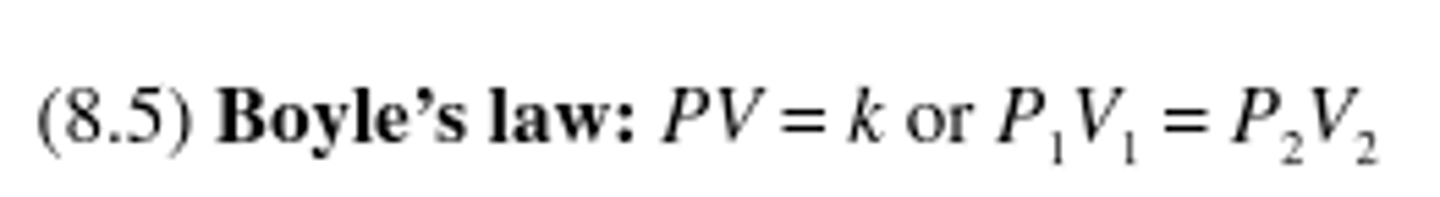
Charle's law
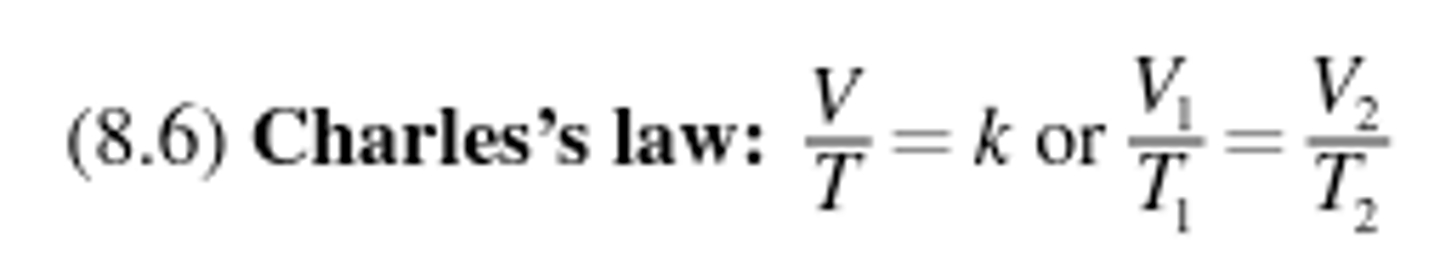
Gay-Lussac's law
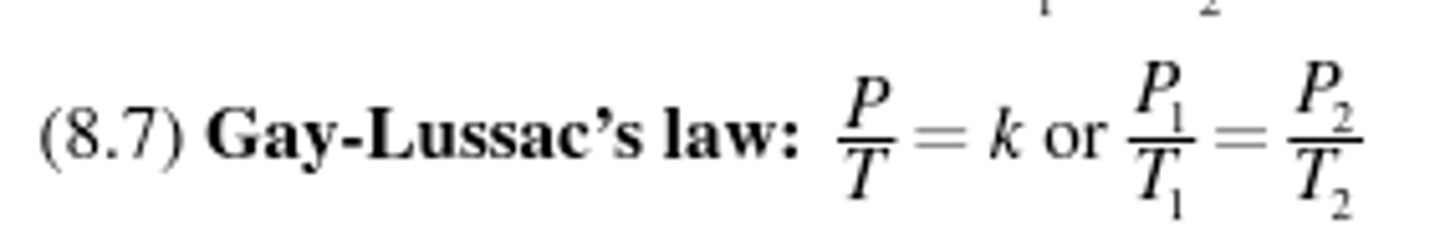
Dalton's law
(total pressure from partial pressure)
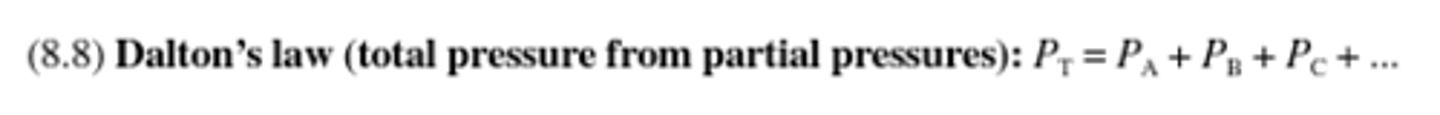
Dalton's law (partial pressure from total pressure)

Henry's law equation
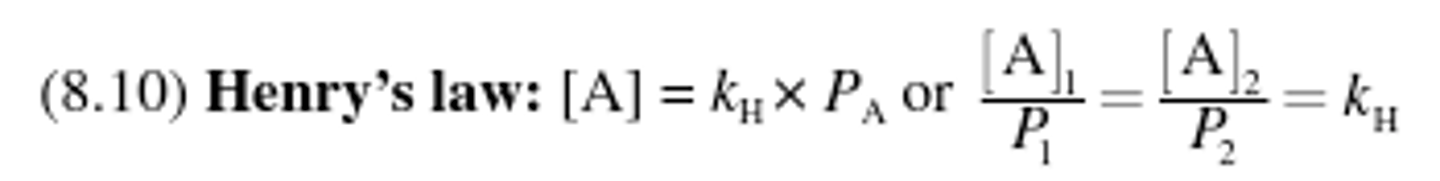
vapor pressure
pressure exerted by evaporated particles above the surface of a liquid
Average kinetic energy of a gas
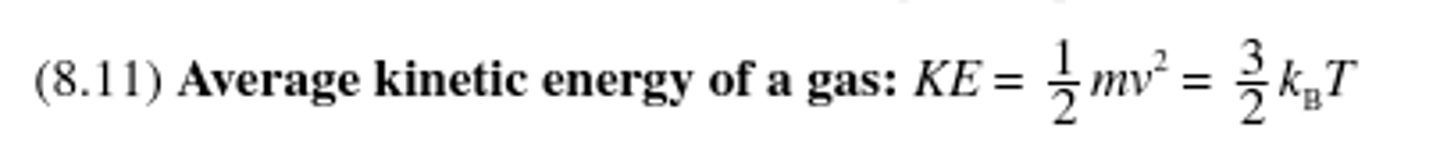
Root-mean squared speed
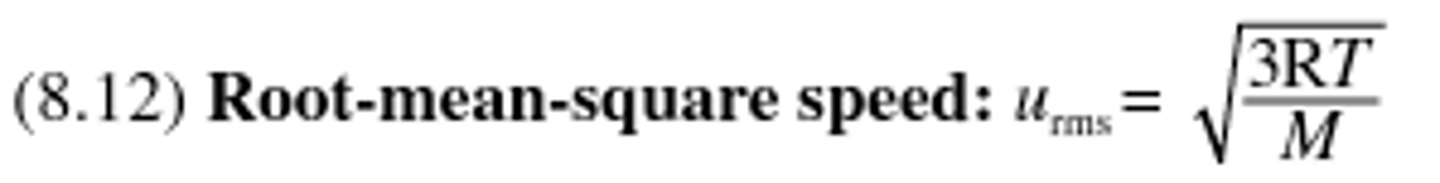
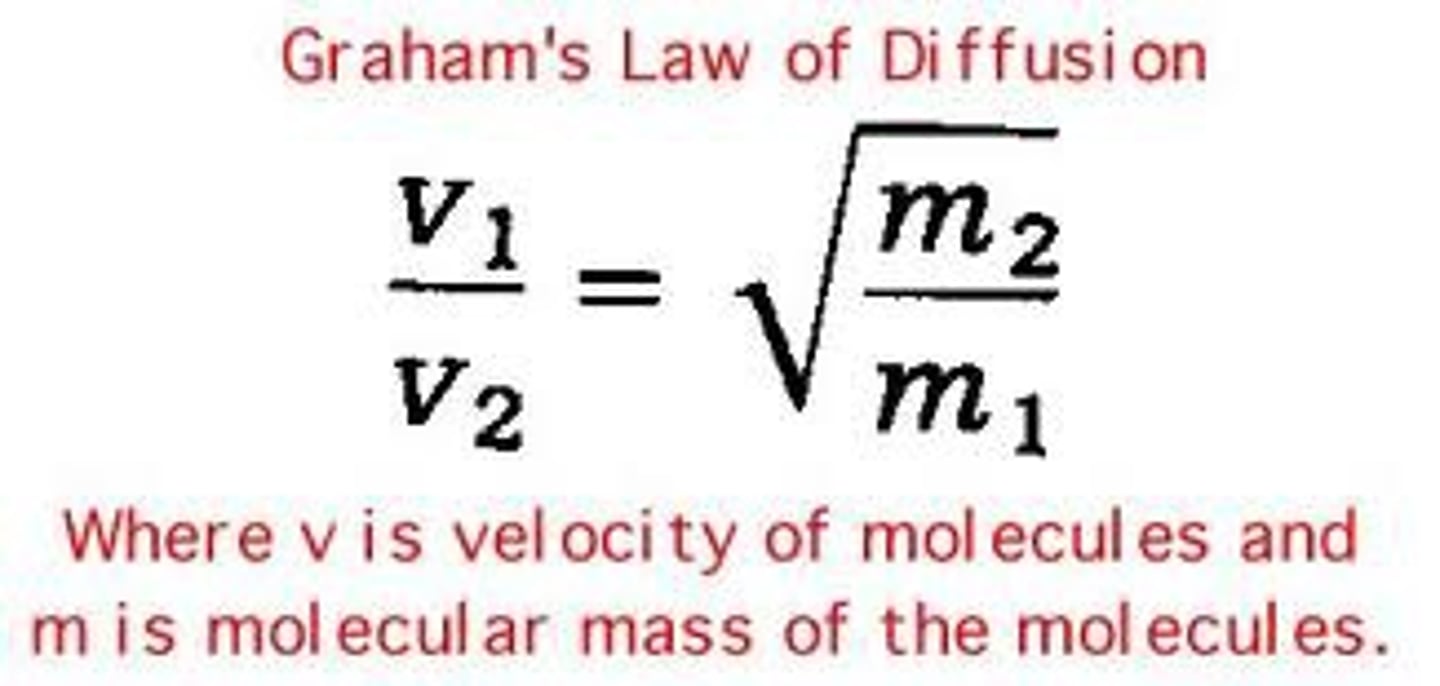
Graham's law equation
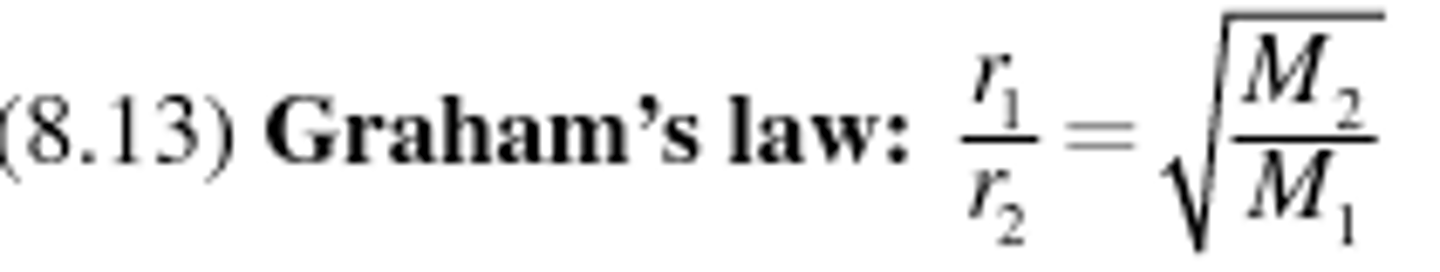
Van der Waals equation of state
Is used to correct the ideal gas law for intermolecular attractions and molecular volume.
a is for attractive forces
b is for big particles
if a and b are both 0, then the van der Waals equation of state reduces to the ideal gas law
(don't need to memorize this equation)

Gaseous state defined by 4 variables:
(variables)
We can define the state of a gaseous sample by four variables:
1) Pressure (P)
2) Volume (V)
3) Temperature (T)
4) # of Moles (n)
Gas Pressure (units?)
Expressed in:
1 ATM = 760 mmHg (equv. to torr) = 100 kPa
The SI unit for pressure is the pascal (Pa)
Standard temperature and pressure (STP)
The conditions under which the volume of a gas is usually measured:
1) 273 K or 0 C.
2) 1 ATM or 101 kPa
1 mole ideal gas = 22.4 L space
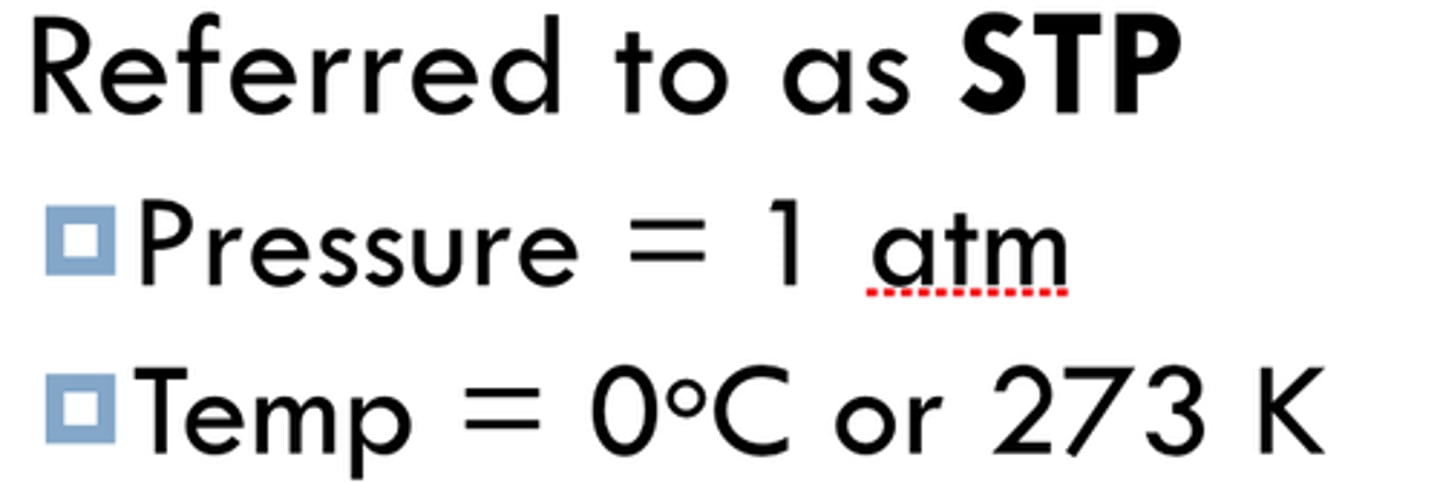
STP vs Standard State Conditions
STP = gas law calculations (1 atm + 273K)
Standard state conditions = enthalpy, entropy, free energy changes, and electrochemical voltage ( 298 K and 1M concentrations).
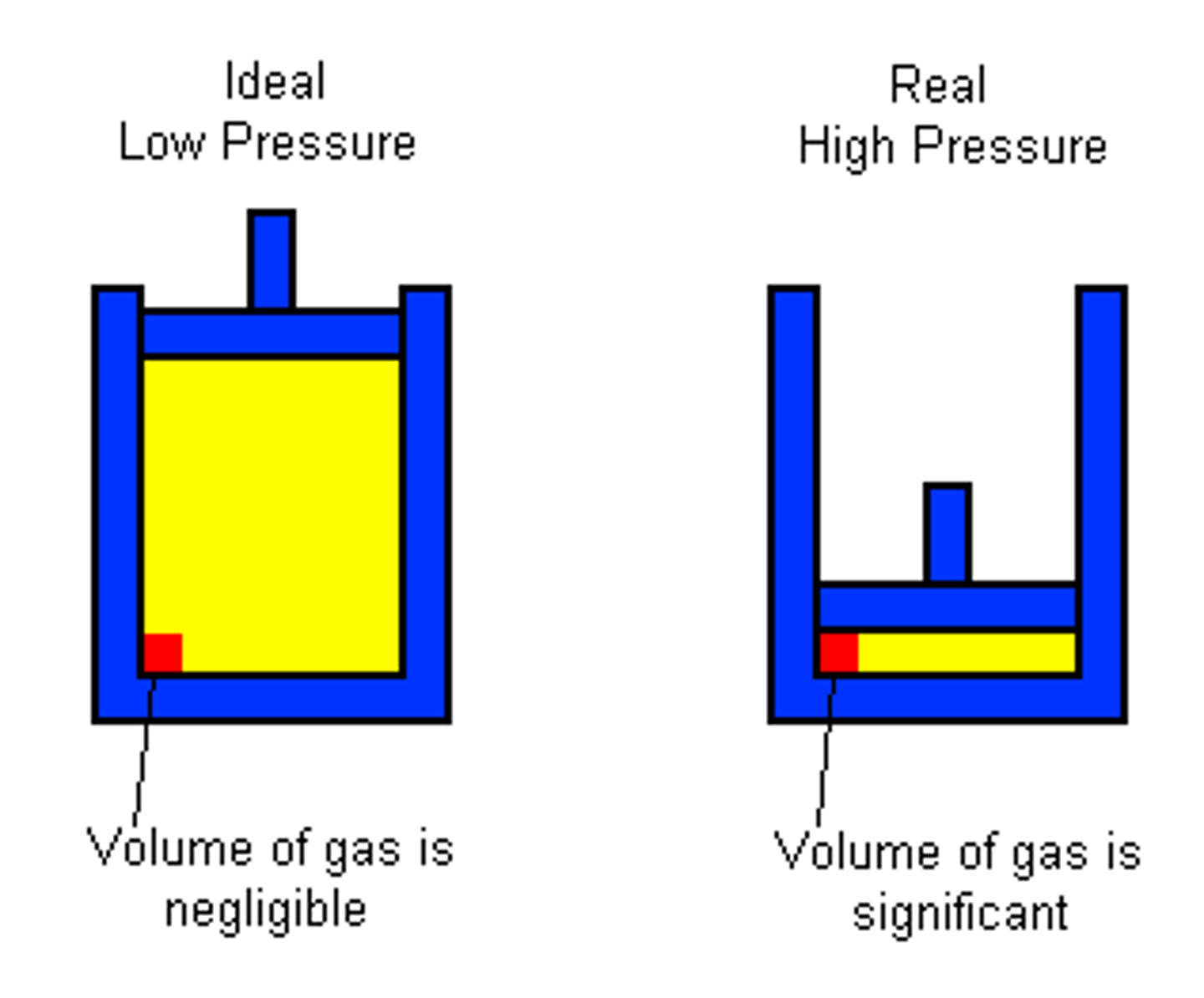
Ideal gas
A hypothetical gas that has no intermolecular forces and occupies no volume.
Although real gases deviate from this idea at high temp or low volume, it is still a close estimate
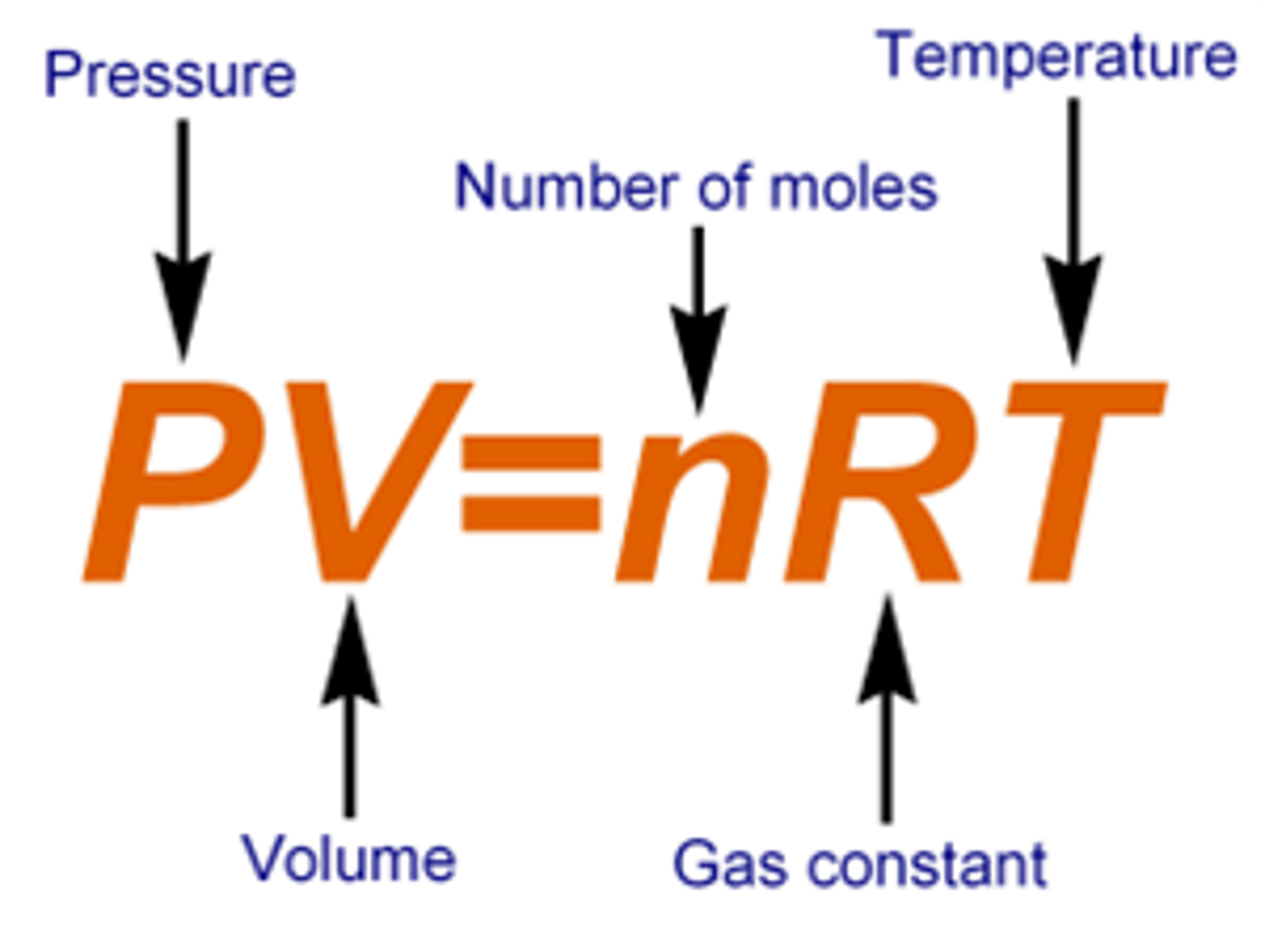
Ideal gas law
Shows the relationship among four variables that define a sample of gas:
PV=nRT
R is the ideal gas constant, which is 8.21x10^-2
and has units of ( L x atm / mol x K)
or may also be seen as 8.314 ( J / K x mol)
Ideal gas constant
Use 0.08 when using atm in numerator!

When to use the Ideal gas law?
The ideal gas law can be used to determine the missing term when given all the others. It can also be used to calculate a change in the term holding two of the others constant.
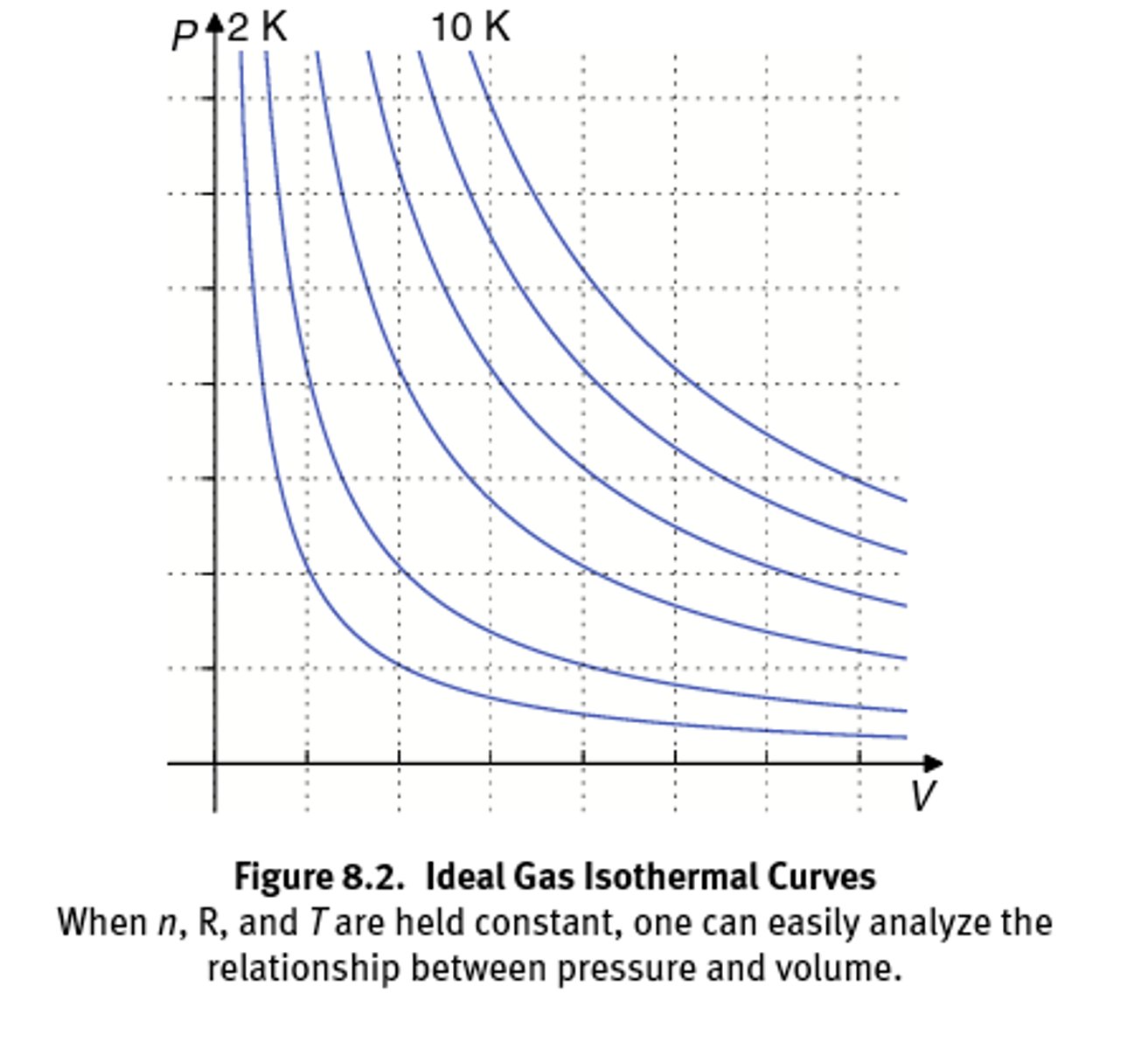
Density of a gas
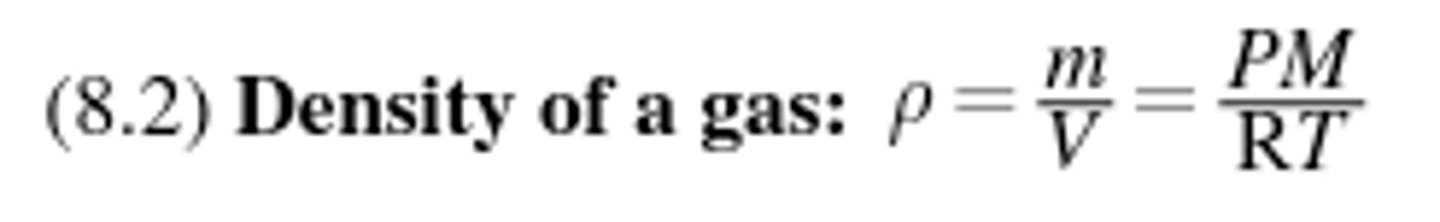
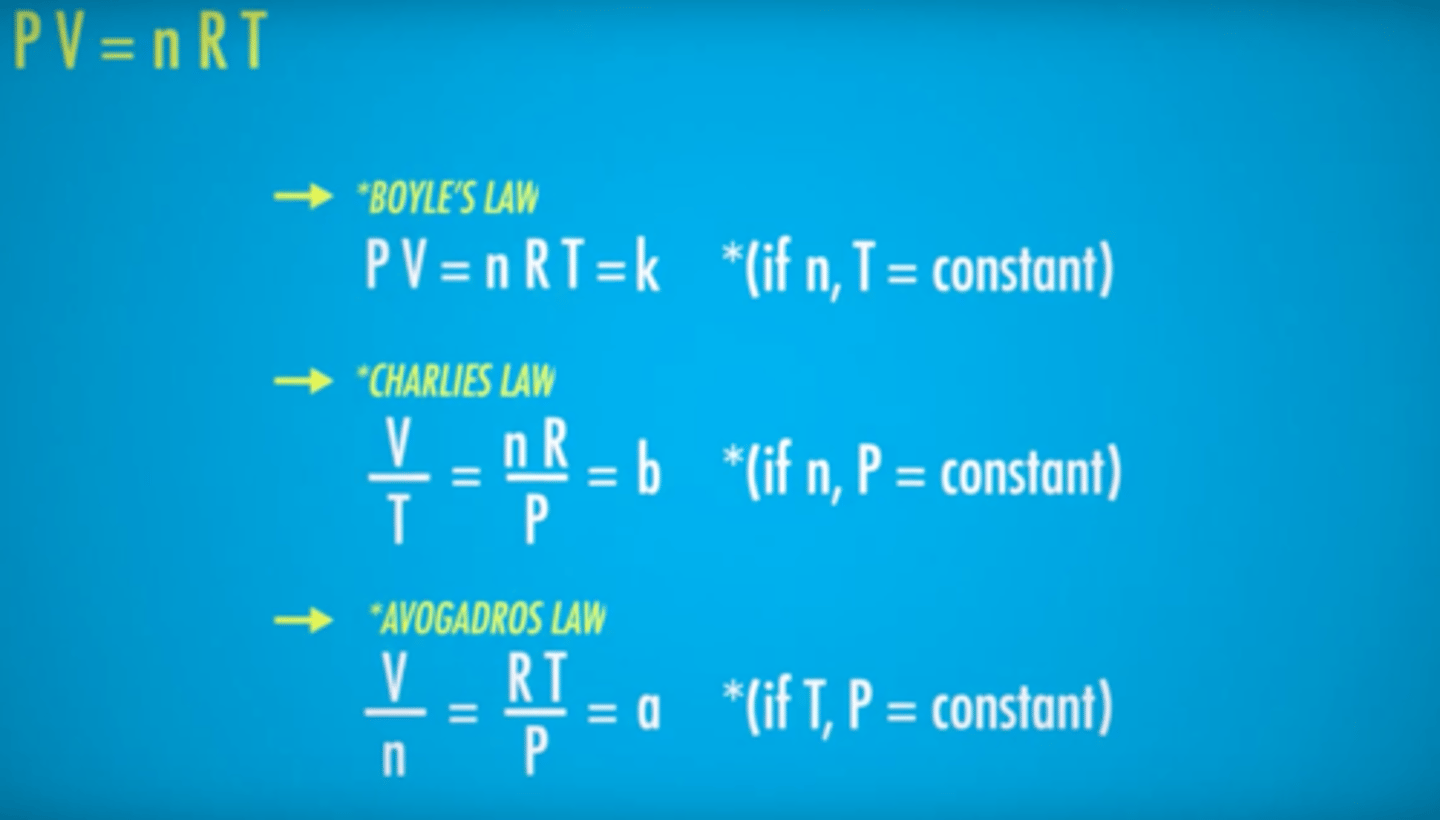
Avogadro's Principle
All gases at a constant pressure and temperature occupy volumes that are directly proportional to the number of moles of gas present.

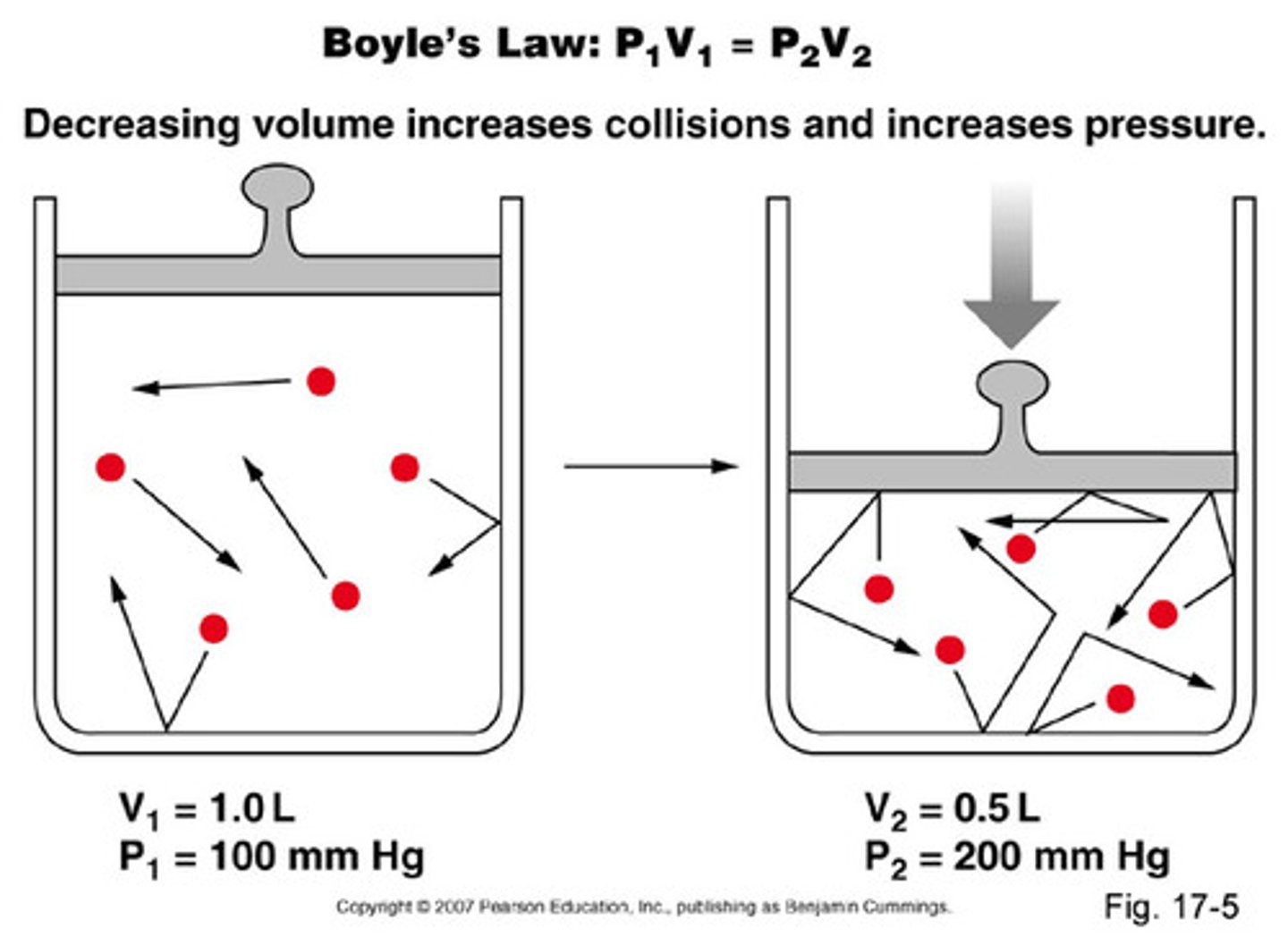
Boyle's Law
For a given gaseous sample held at constant temperature (isothermal conditions), the volume of the gas is inversely proportional to the pressure.
Boyle's law graph
as pressure increases, the volume decreases and vice versa
Charles law
States that at constant pressure, the volume of a gas is proportional to its absolute temperature.
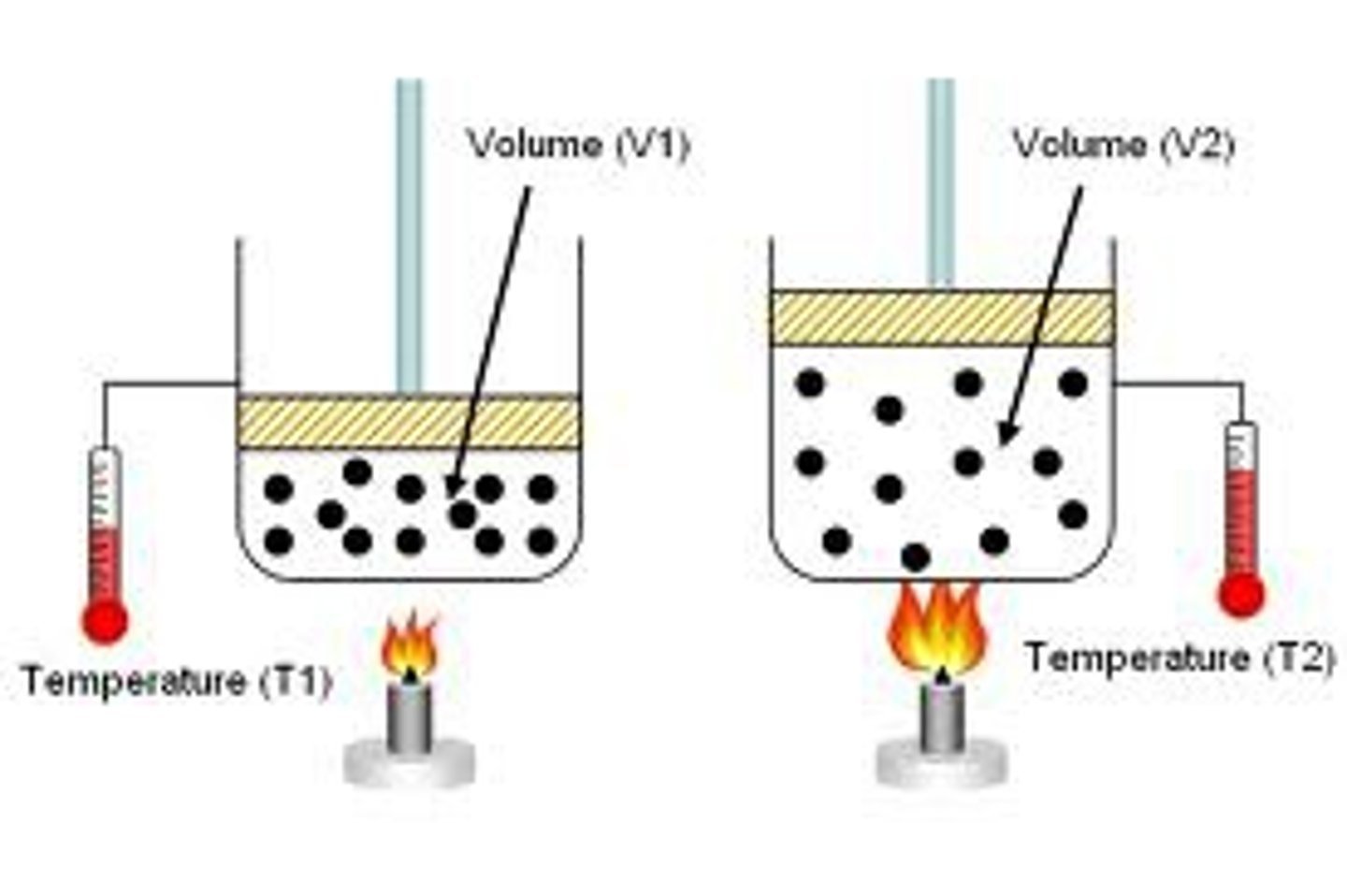
Charles law graph
volume and temp are directly proportional: when one increases, the other increases in direct proportion
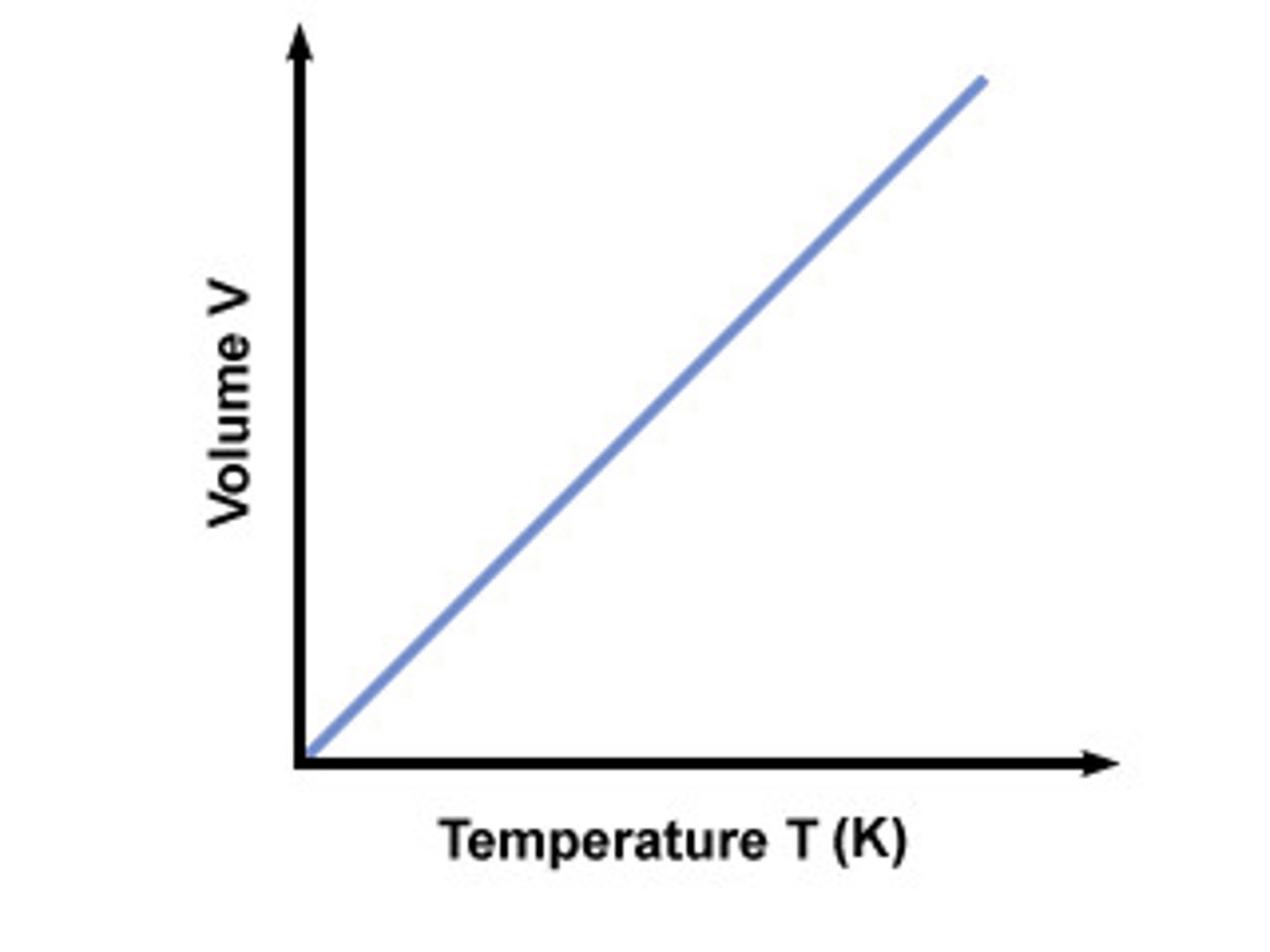
Gay-Lussac's Law
Relates temperature and pressure
Ideal gas law
(related to other laws)
Dalton's law
States that the total pressure of a gaseous mixture is equal to the sum of the partial pressures of the individual components.
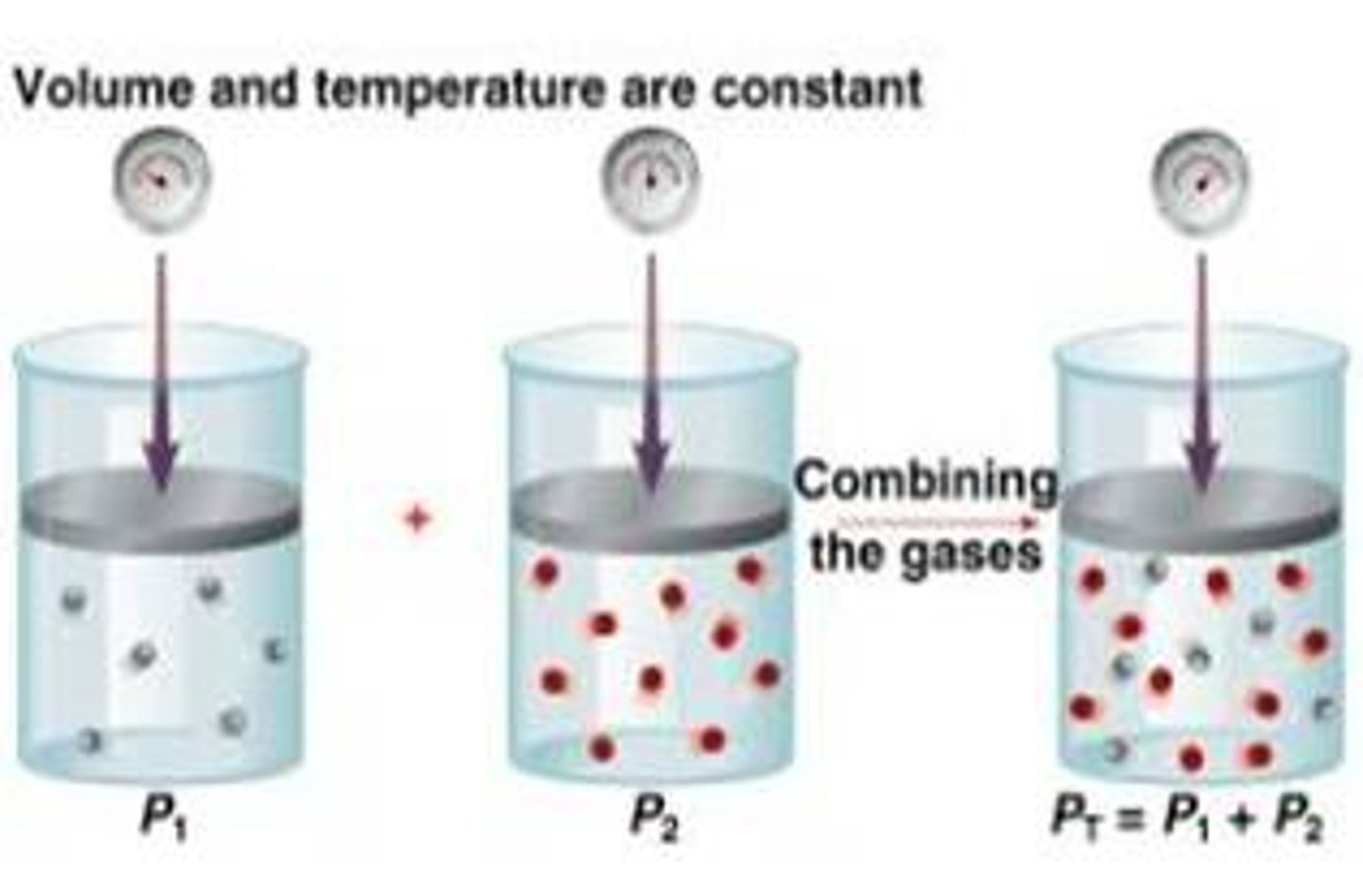
Partial pressure
the contribution each gas in a mixture of gases makes to the total pressure

Henry's law
States that the amount of gas dissolved in solution is directly proportional to the partial pressure of that gas at the surface of solution.
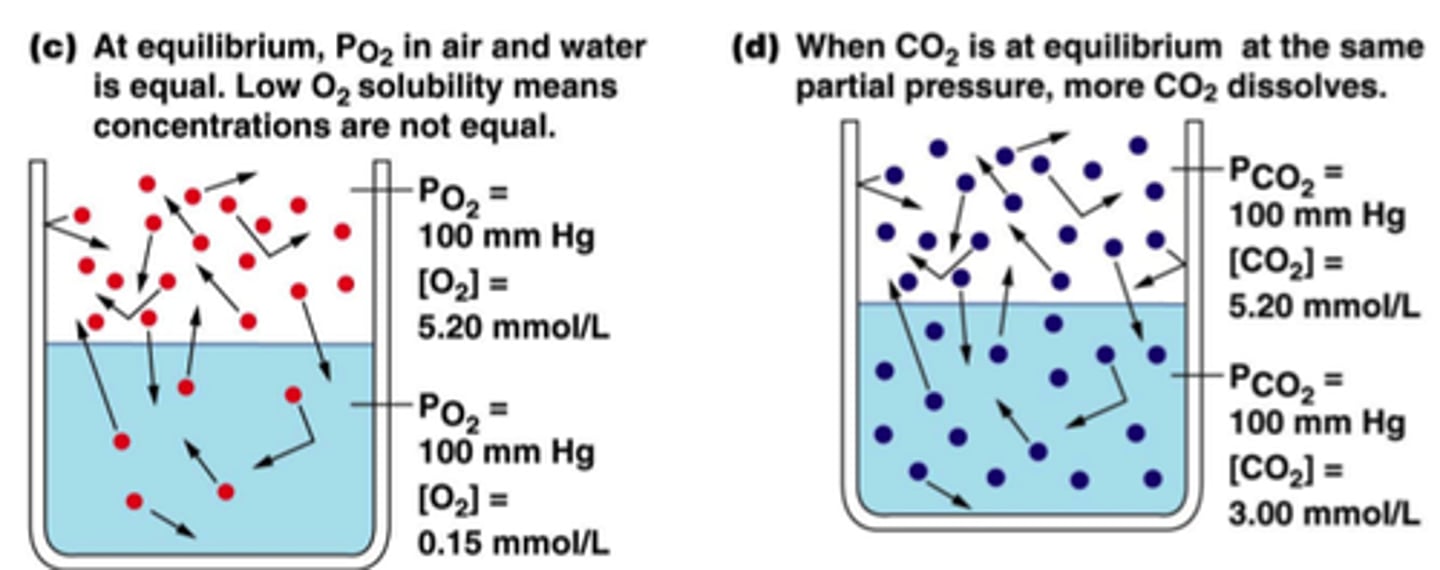
The solubility of a gas will increase with ____________ partial pressure of the gas
The solubility of a gas will increase with __increasing__ partial pressure of the gas
Kinetic molecular theory
Attempts to explain the behavior of gas particles. It makes a number of assumptions about them:
1) Gas particles have negligible volume
2) No intermolecular forces
3) Undergo random collisions with them + walls of container
4) Avg. kinetic energy of gas particle is directly proportional to temp.
The higher the temp, the _________ the molecules move
The larger the molecules, the ________ they move
The higher the temp, the __faster__ the molecules move
The larger the molecules, the __slower__ they move
Graham's law
Describes the behavior of a gas diffusion or effusion, stating that gases with lower molar masses will diffuse or effuse faster than gases with higher molar masses at the same temperature.
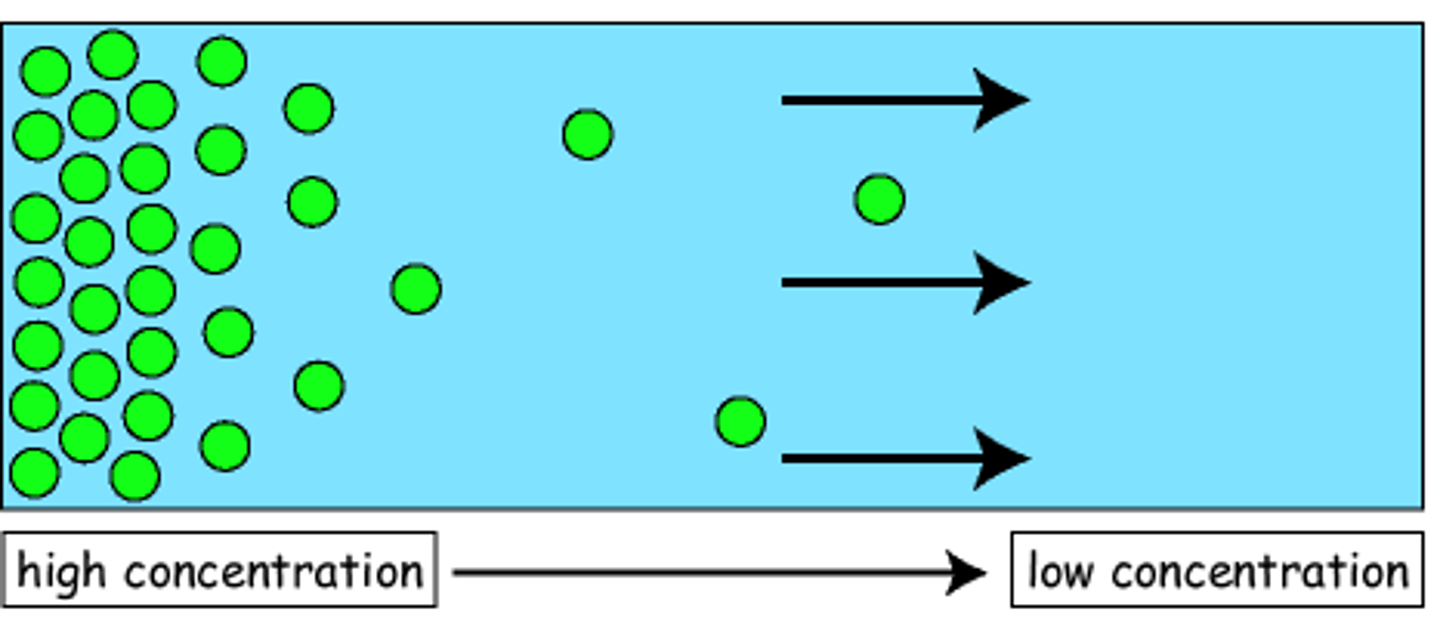
Diffusion
The spending out of particles from high concentration to low concentration
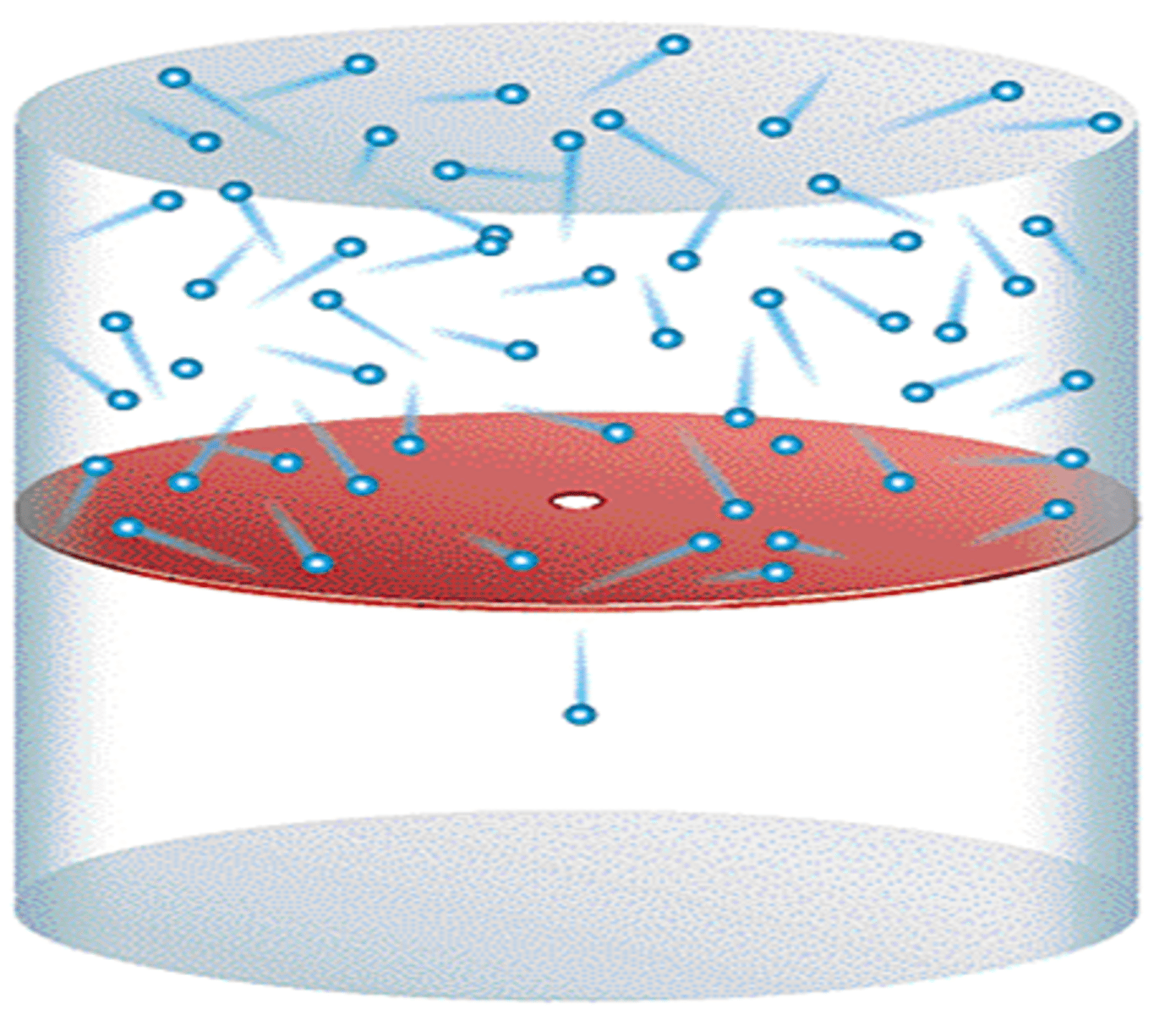
Effusion
The net movement of gas under pressure from one compartment to another through a small opening
Real gases
Deviate from ideal behavior under high pressure (low volume) and low temperature conditions.
1) At moderately high pressure, low volume, or low temperature, real gases will occupy more volume than predicted by the ideal gas law because the actual particles occupy physical space.
2) At extremely high pressure, low temperature, real gases will occupy more volume than predicted by the ideal gas law because particles occupy physical space.
gas near boiling point
intermolecular attraction causes the gas to have a smaller volume than that which would be predicted by the ideal gas law
loser it gets to boiling point, less ideally it acts
at extremely low temperatures,
gases will again occupy more space than predicted because particles cannot be compressed to zero volume
Ideal gases:
I. have no volume
II. have particles with no attractive forces between them
III. have mo mass
II only, (gases have volume, individual particles do not)
an 8.00 g sample of NH4NO3 is placed into an evacuated 10L flask and heated to 227 degrees celsius. After the NH4NO3 completely decomposes, what is the approximate pressure in the flask?
NH4NO3 --> N2O + H2O
BALANCE THE EQUATION,
NH4NO3 --> N2O + 2H2O
mass given in 8g so molar mass is .1mol because mass of NH4NO3 is 80 g/mol. so when .1 mol decomposes you get .1 mol N2O and .2 mol water based on stoic so this gives .3 mols gas product. use ideal gas caution to get pressure
P=.3 x .0821 x 500/ 10 = 1.2 atm
experimenters notice that the molar concentration of dissolved oxygen in an enclosed water tank has decreased to one-half its original value. in an attempt to counter this decrease, they quadruple the partial pressure of oxygen in the container. what is the final concentration of the gas?
c. double the original
solubility directly proportional to pressure so times 4 is times 4 but we have .5 so its .5 times 4 is double