MICI 3115 Lecture 1 - Cells and Organs of the Immune System
1/68
Earn XP
Description and Tags
Flashcards covering the cells, organs, and processes of the immune system, including hematopoiesis, leukocyte differentiation, and the functions of various immune cells and lymphoid tissues.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
What is the immune system's primary role in the body?
To maintain homeostasis through a complex system of interacting cells and molecules (redundant features working together to generate response through activation & resolution)
What are 'sentinel cells' in the immune system?
Cells present (often resident) within tissues with immunologic function, varying by location and cell type.
List some functions of sentinel cells.
Direct detection and elimination of pathogens, recruitment of other immune cells via cytokines/chemokines, early polarization of immune responses, phagocytosis, and antigen presentation.
Cytokine
Diverse group of immune-modulating (messengers) proteins that are broadly involved in regulating the immune system, inflammation, cell growth, and antibody-mediated immunity.
Ex: interferons (IFNs), interleukins (ILs), tumour necrosis factors (TNFs) & colony stimulating factors (CSFs)
Chemokine
A specific type of cytokine whose primary function is to direct the chemotaxis (movement) of immune cells, such as white blood cells, to sites of infection or injury in the body.
Important: All chemokines are cytokines, but not all cytokines are chemokines
Where are 'circulating/ready-made effector cells' found?
In lymphoid tissues or circulation in the absence of infection.
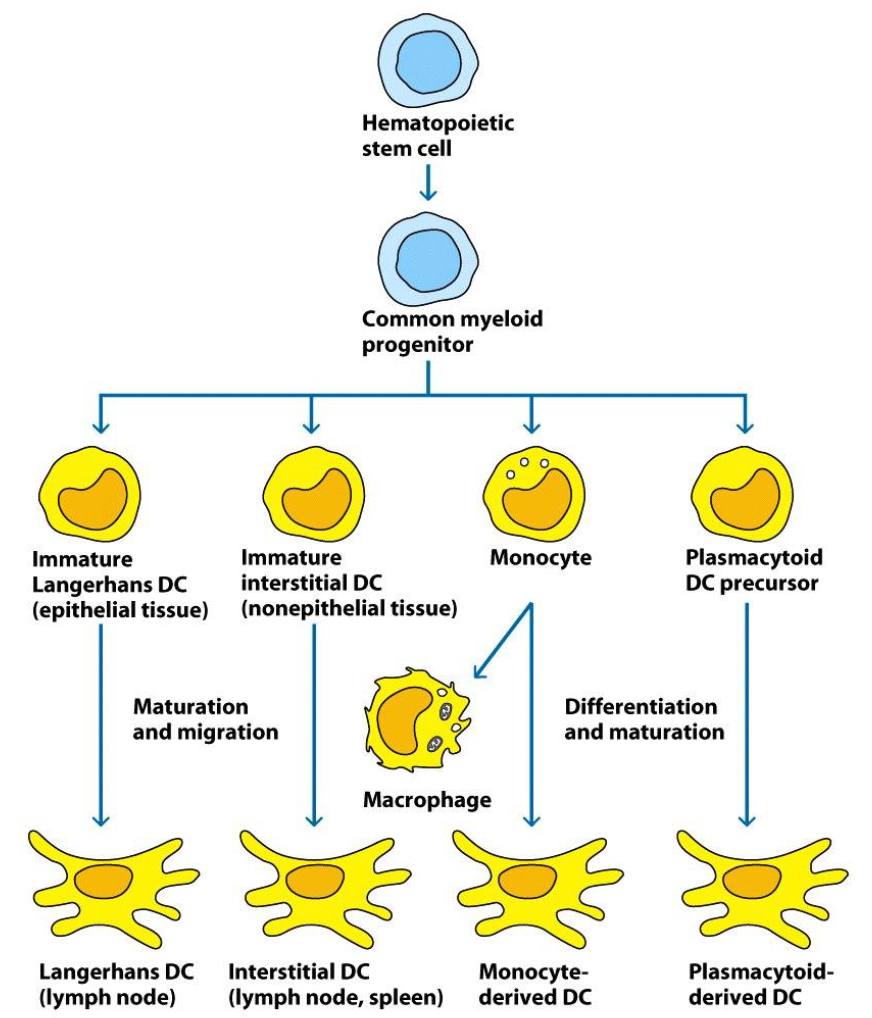
What is hematopoiesis?
The formation and development of both red blood cells (erythrocytes) and white blood cells (leukocytes) from self-renewing, pluripotent hematopoietic stem cells (HSC).
Name the three major lineages of cells derived from hematopoietic stem cells (HSC).
White blood cells (granulocytes, monocytes, lymphocytes), red blood cells (made - erythropoiesis), and platelets (made - thrombopoiesis).
What process yields granulocytes (WBC)? Can you name the 4 types?
Granulopoiesis
Neutrophils, eosinophils, basophils, mast cells
What process yields monocytes? Name all 3 cells.
Monopoiesis
Monocytes, macrophages, and dendritic cells
What process yields lymphocytes? Name the 5 cell types.
Lymphopoiesis
T cells, B cells, NK cells, ILC and NKT cells
Where does hematopoiesis primarily occur in adults?
Vertebrae, ribs, sternum, skull, sacrum, pelvis, and the ends of femurs.
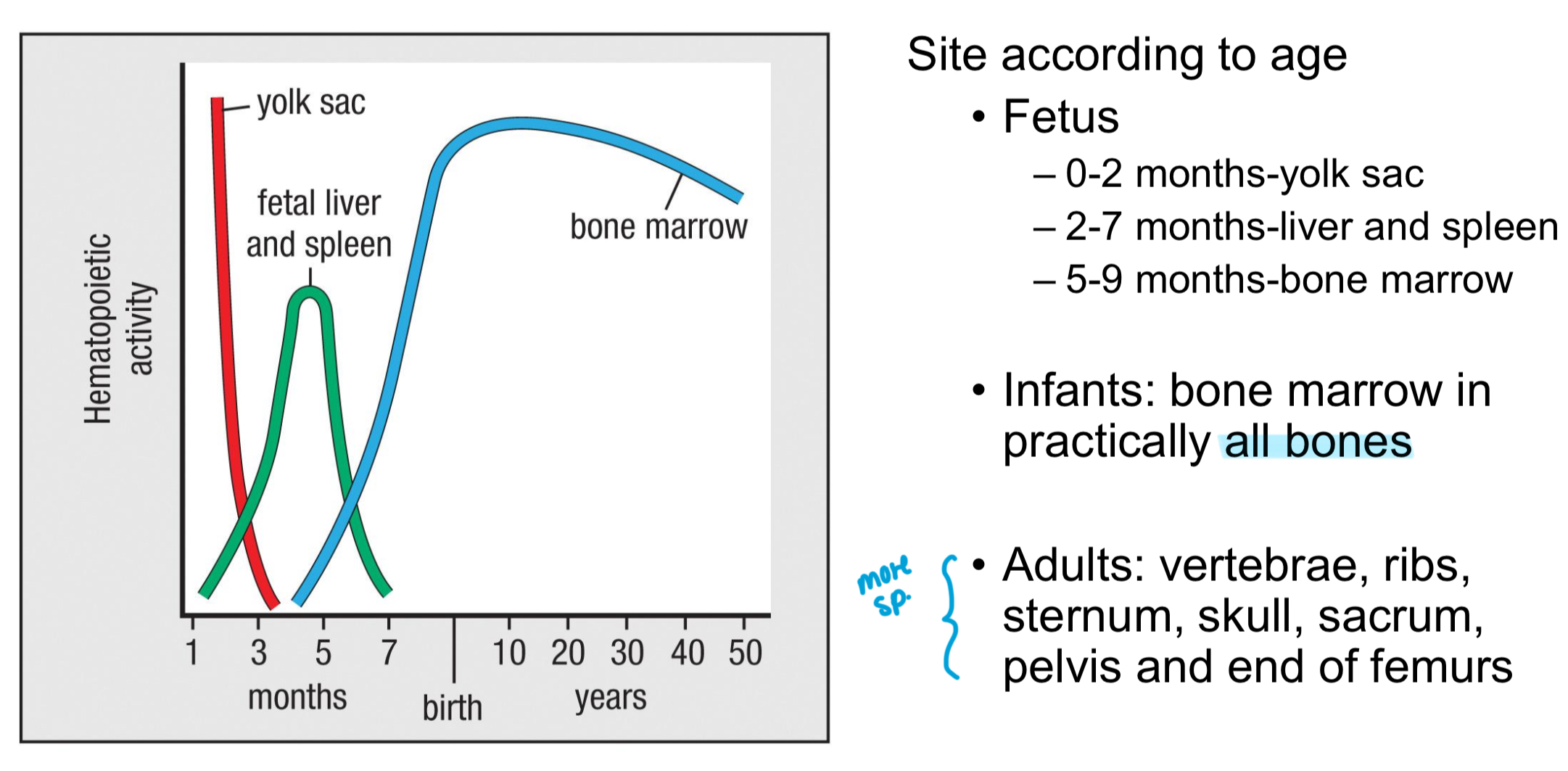
Which lymphoid tissue is involved in the production of leukocytes and acts as a primary lymphoid tissue?
The bone marrow.
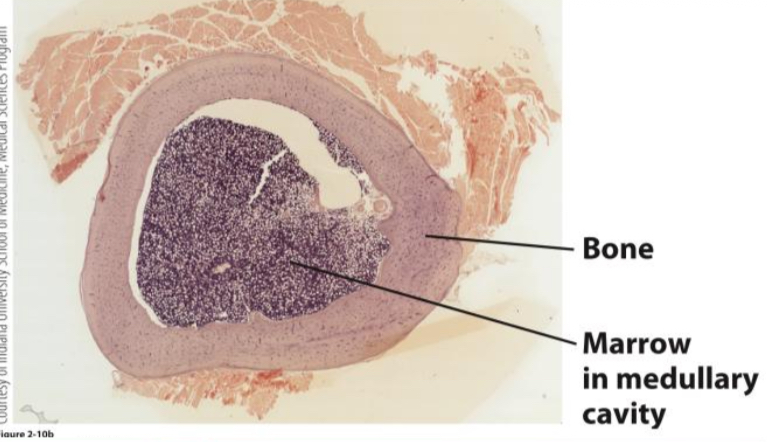
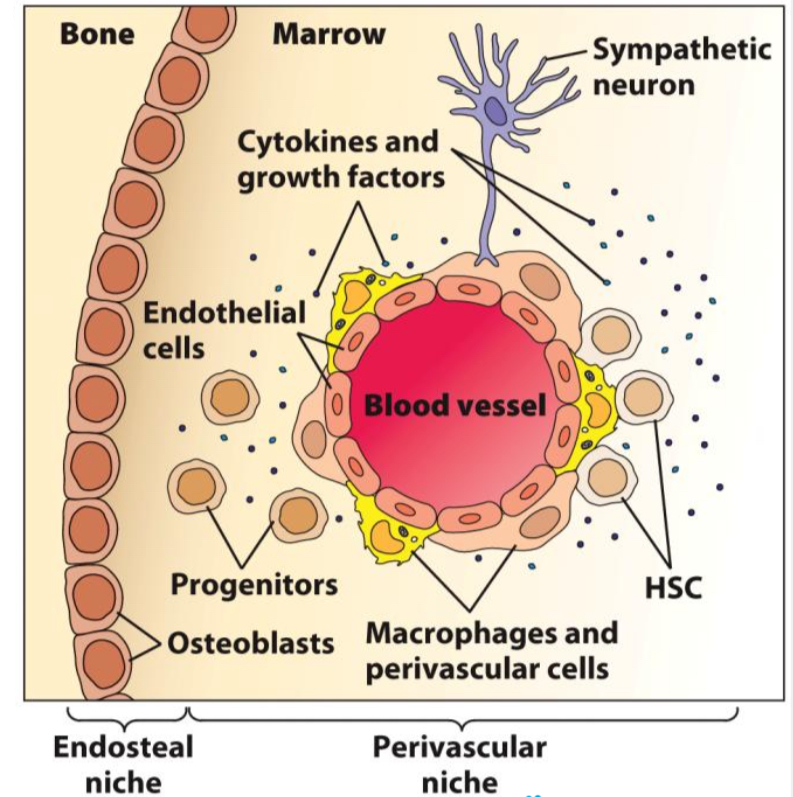
What are the major components of the bone marrow microenvironment (niche)?
Extracellular matrix
stem cells & hematopoietic progenitor cells attached by adhesion molec.
Stromal cells
endothelial, perivascular, nerves, macrophages and osteoblasts
scaffold for growth of hematopoietic cells
nutrients, produce hematopoietic growth factors and express adhesion molecules influence differentiation
Hematopoietic growth factors
presented to immobilized stem cells
Leukocytes differentiate from hematopoietic stem cells in bone marrow into which two main lineages?
Myeloid or lymphoid lineages.
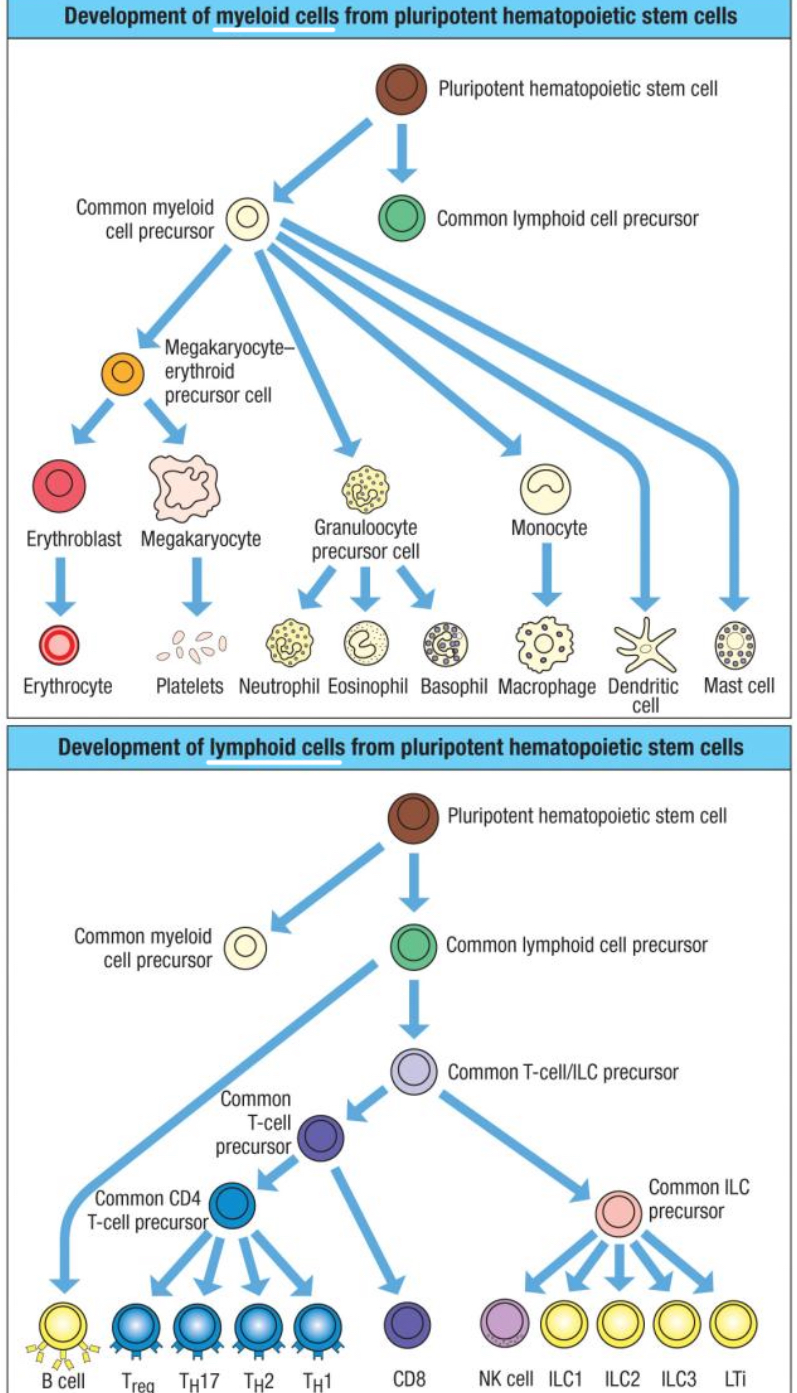
What cells are in the myeloid lineage?
Granulocytes (neutrophils, eosinophils, basophils, mast cells) and monocytes (monocytes, macrophages, dendritic cells).
Where do T lymphocytes mature?
Thymus
Where does additional leukocyte maturation and activation occur?
Periphery, in response to stimulation, growth, and tissue-resident factors.
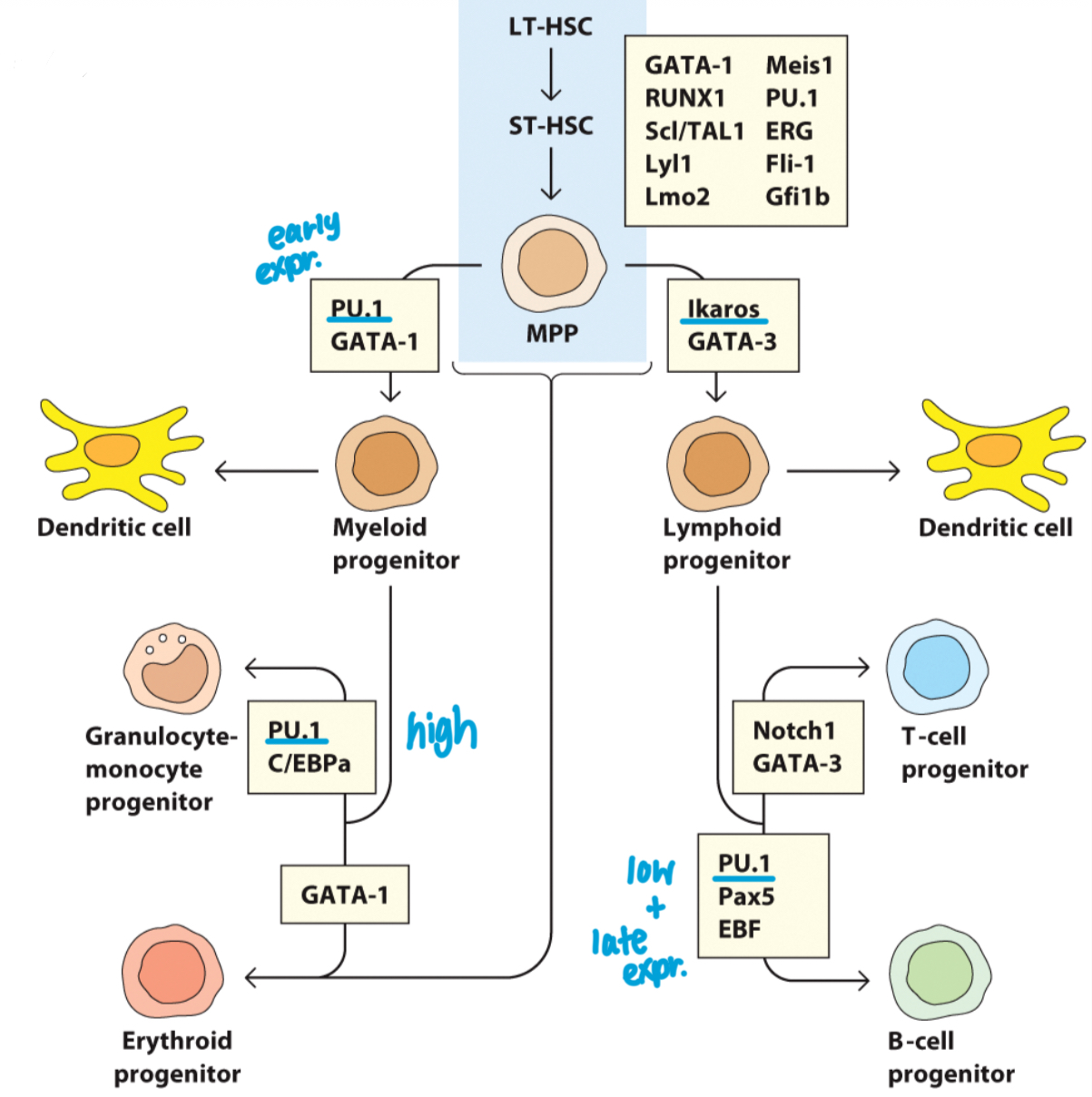
Which transcription factor drives the differentiation of lymphocytes and shuts down the myeloid lineage fate?
Ikaros.
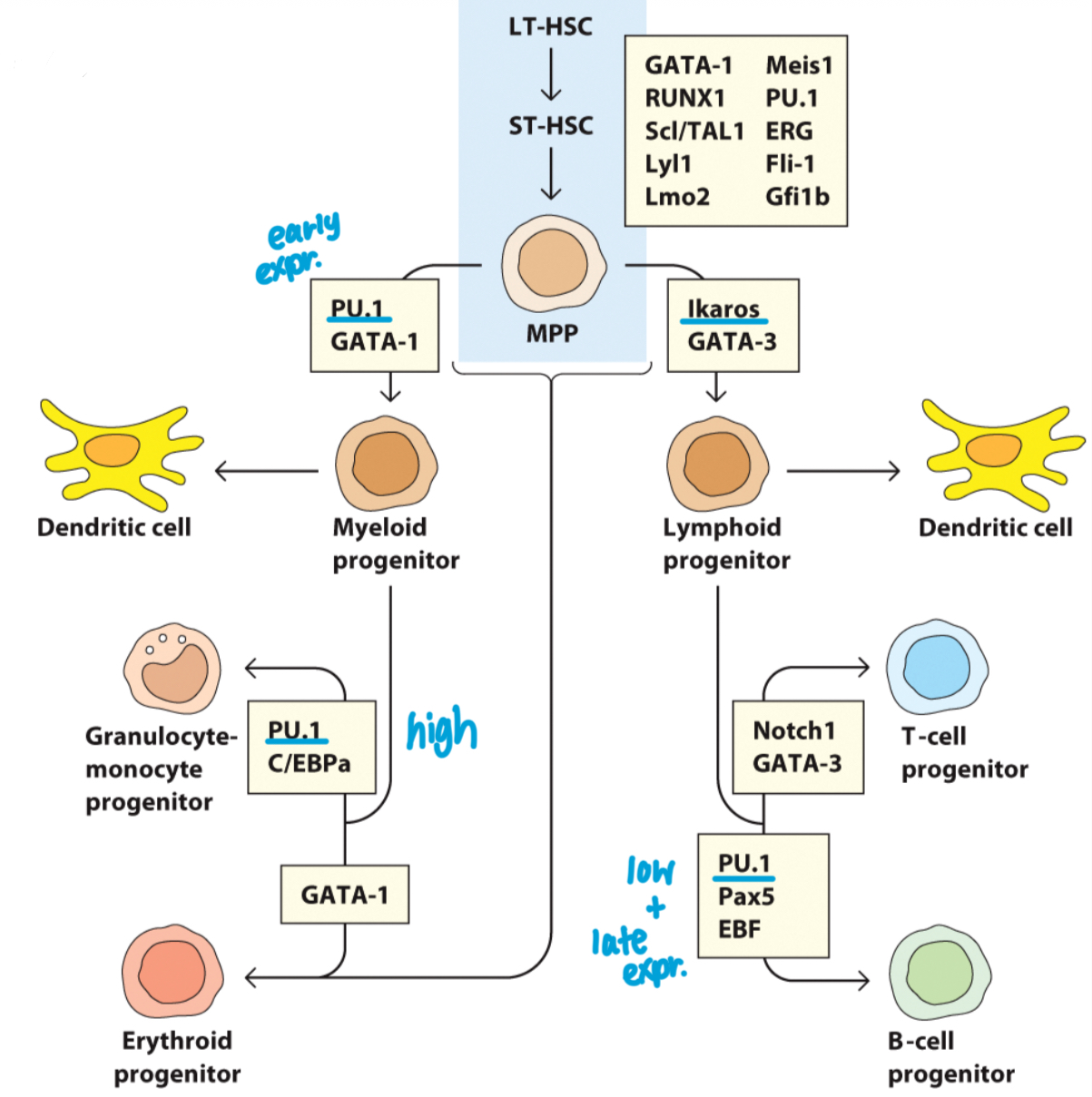
How does PU.1 expression influence a developing cell's fate?
Low PU.1 leads to a lymphoid fate, while high PU.1 leads to a myeloid fate.
Overall: Pluripotency maintenance in hematopoietic stem cells (HSCs) is controlled by an array of factors.
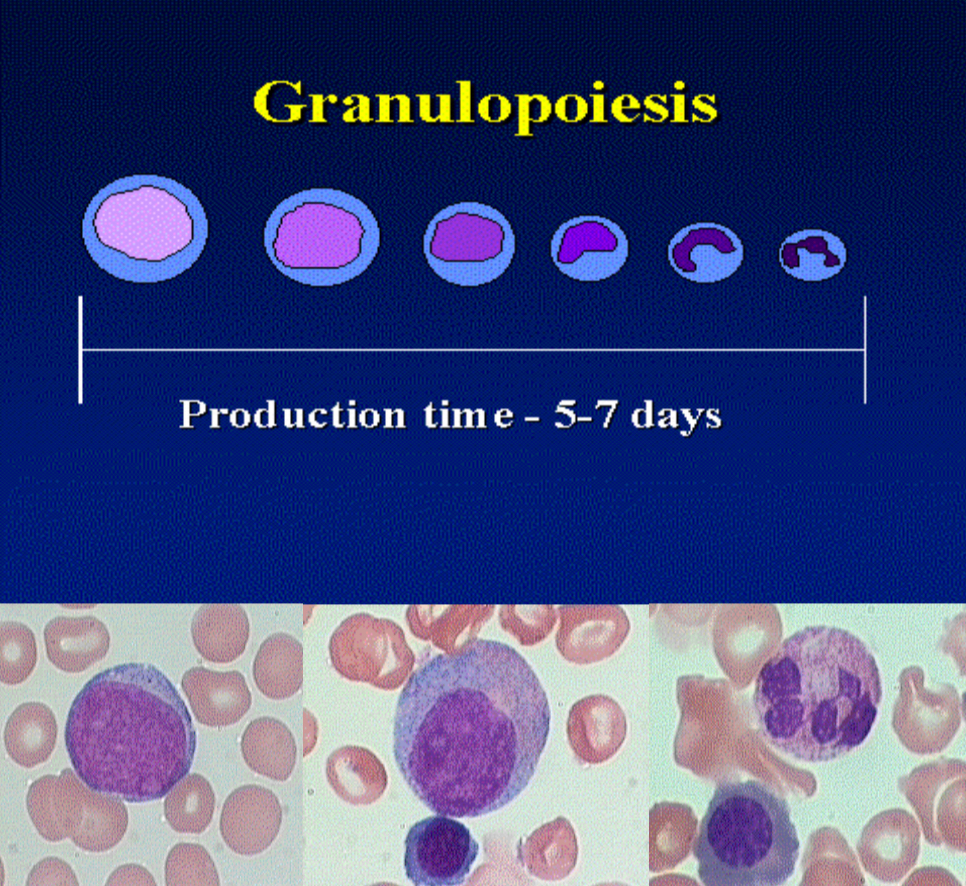
What are the key regulatory factors for granulopoiesis?
Interleukin (IL)-3 and Granulocyte-macrophage-colony stimulating factor (GM-CSF)
G-CSF - neutrophils
IL-4 - basophils and eosinophils
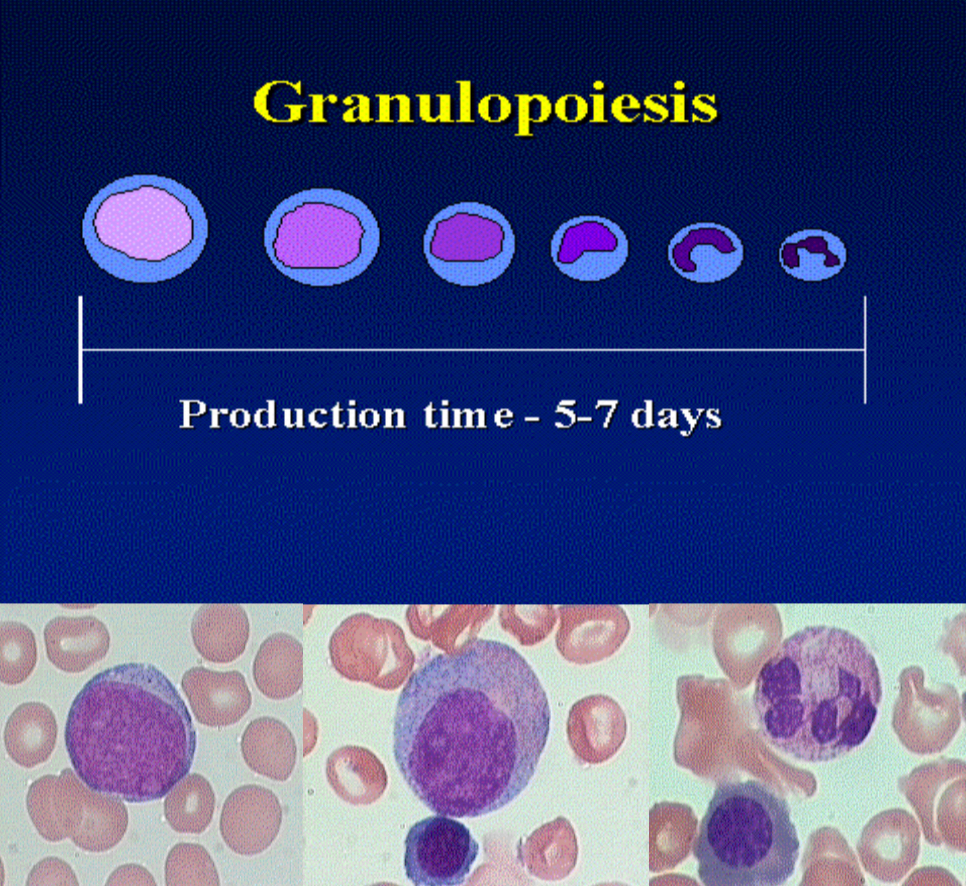
What are key characteristics of granulopoiesis maturation?
Nuclear segmentation
Acquisition of primary, then secondary granules
Negative feedback by mature granulocytes
Formation rate: 1–2 × 10⁹ granulocytes/kg/day
List the general features of granulocytes.
Very early responders to infection from array of extracellular pathogens (minutes to 48h), not antigen-specific, derived from the myeloid lineage, classified by morphology and granule staining, and release products to recruit other immune cells.
What is the most abundant leukocyte in circulation? Describe its morphology.
Neutrophils (50-70%)
Circulate 7–10 hrs; survive ~48 hrs in tissues
1st responders in infection, swarm where chemokines located
If a lot in blood indicative of infection
Morphology
Segmented nucleus (3–4 segments)
Primary granules: large, antimicrobial
Secondary granules: contain complement activators, enzymes
Tertiary granules: contain phosphatases, metalloproteinases
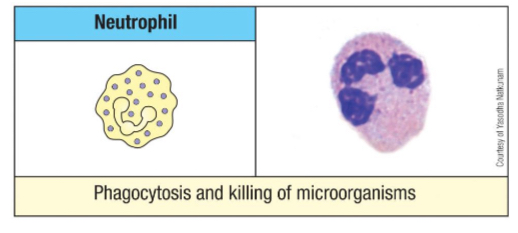
What are the primary functions of neutrophils?
Phagocytosis of bacteria and debris, release of chromatin to trap microbes (NETosis), secretion of proteins to kill bacteria and signal tissue remodeling, and assisting in shaping adaptive immune responses.
Dead ones accumulate as pus on wound
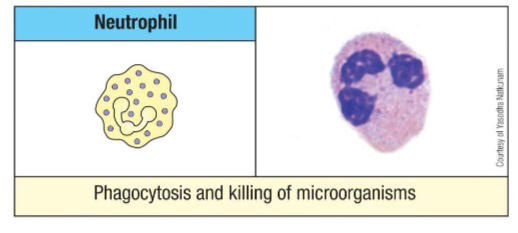
Name the two major approaches neutrophils use for killing ingested bacteria.
Oxygen-dependent (mitochondria)
Reactive oxygen intermediates (O2-, OH, H2O2, ClO-)
Reactive nitrogen intermediates (NO)
Oxygen-independent (granules)
Lysozyme
Defensins
Hydrolytic enzymes
Tumor necrosis factor
Give an example of a molecule found in Neutrophil granule and its function.
Proteases
Elastase, collagenase = tissue remodeling
Antimicrobial peptides
Defensins, lysozymes = harm pathogens
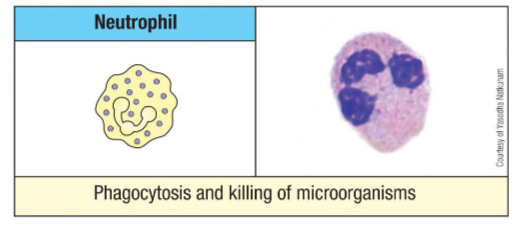
Eosinophils are especially prevalent in which body location?
The small intestine.
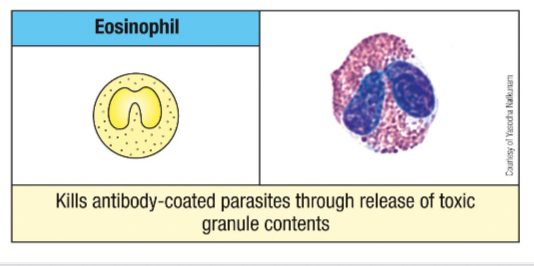
What are the primary functions of eosinophils?
Anti-parasitic function, involvement in allergy and asthma, and release of cytokines to instruct adaptive immune responses, especially against multicellular parasites.
Use lysosomal enzymes & oxygen radicals
Anti parasite protein - eosinophil cationic protein (ECP)
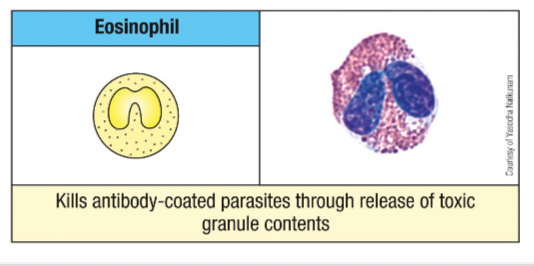
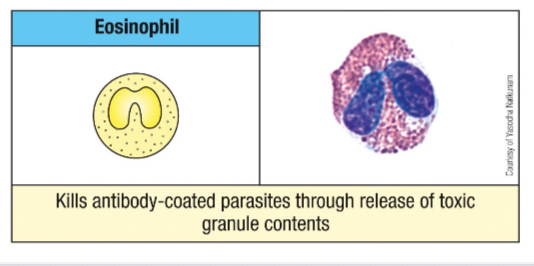
Give an example of a molecule found in Eosinophil granule and its function.
Ribonucleases
ECP and EDN - antiviral activity
Cytokines
IL-4, 10 and 13 and TNF-α - modulation of adaptive immune response
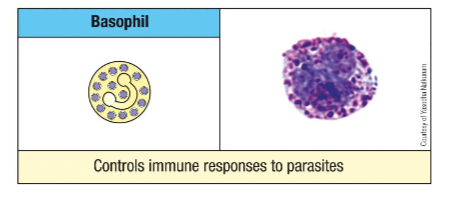
What is the frequency of basophils in circulation? Describe their morphology.
<1% of leukocytes.
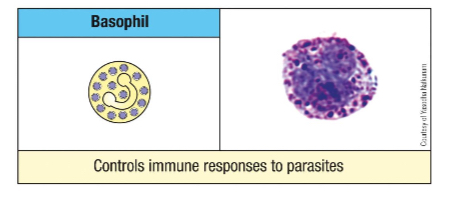
Give an example of a molecule found in Basophil/mast cell granule and its function.
Cytokines
IL-4 and 13 - modulation of adaptive immune system
Histamine
Vasodilation and smooth muscle activation
How do basophils contribute to immunity and allergic reactions?
killing extracellular parasites (multicellular worms)
bind circulating antibody/antigen complexes (aka tagged pathogens)
release granule contents like histamines, leukotrienes, prostaglandins, and cytokines
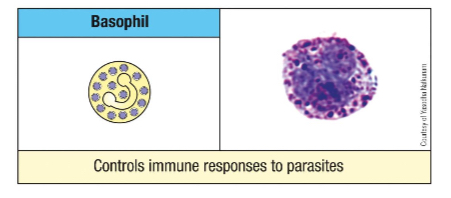
Where do mast cells differentiate after being produced in the bone marrow?
Upon the immature precursors entering tissues, they usually mature at the interface between the body and the environment (e.g., skin, mucosa).
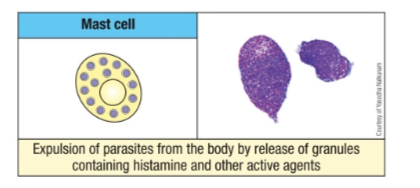
What are the types and roles of mast cells?
Types: Connective tissue & mucosal mast cells
Involved in anti-parasite defense, allergic reactions, and immune modulation
Like basophils, they release granule contents (i.e., histamines, leukotrienes, prostaglandins and cytokines).
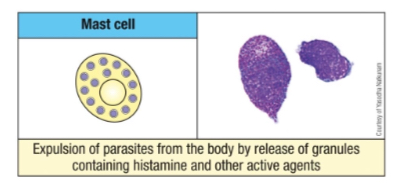
What is the key factor regulating monopoiesis?
Monocyte-colony stimulating factor (M-CSF).
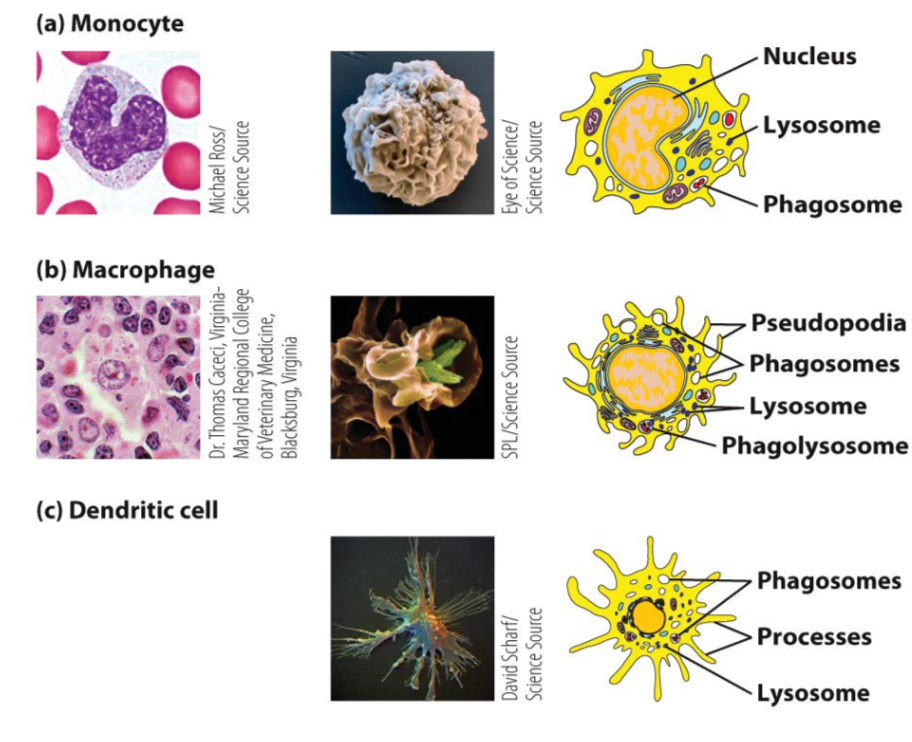
Into what cell types do monocytes further differentiate in tissues?
Dendritic cells and macrophages.
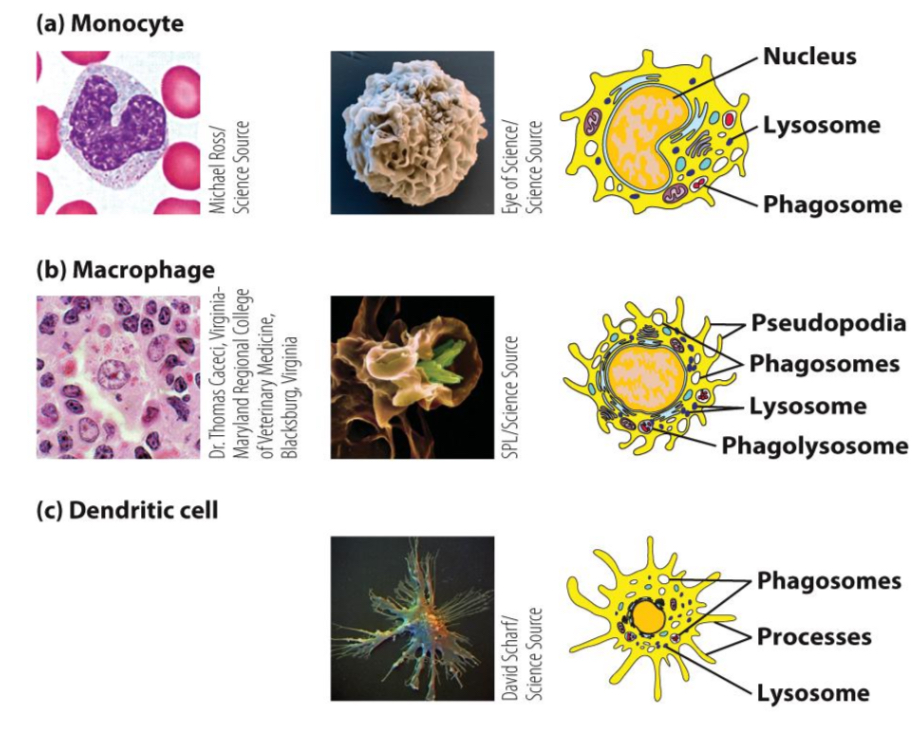
What are the main functions of monocytes and macrophages?
Sentinel function (detect infection, signal immune response)
phagocytosis of microorganisms and apoptotic cells
killing of ingested microorganisms (oxygen-dependent and independent)
recruitment of immune cells (secretion chemokines and cytokines)
antigen presentation to T cells.
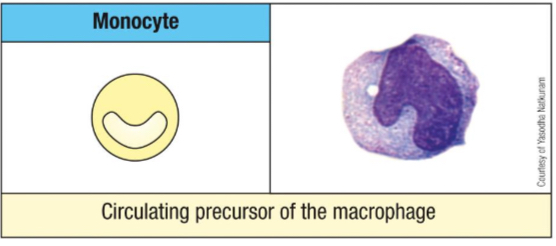
What changes occur when monocytes differentiate into macrophages?
5–10× increase in size
More numerous and complex organelles
Enhanced phagocytic activity
Higher levels of hydrolytic enzymes
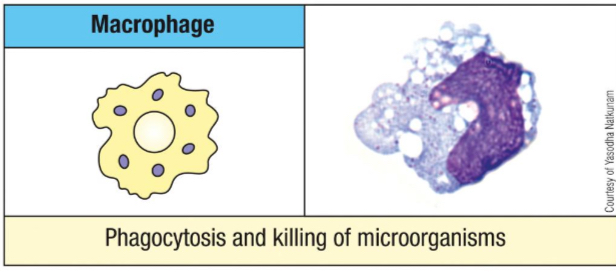
Name three types of macrophages based on their location.
Alveolar (lung)
Kupffer cells (liver)
Microglial cells (brain)
Osteoclasts (bone)
Histiocytes (connective tissues)
Mesangial cells (kidney).
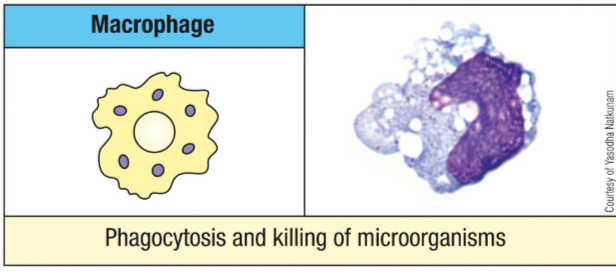
What are macrophages activated by?
phagocytosis
inflammatory Th1 cytokines (IFN-Y) and mediators
bacterial components
What happens to macrophages after activation?
They exhibit enhanced phagocytic activity, increased killing ability, increased secretion of inflammatory mediators, increased migration, and increased ability to activate T cells via antigen-presentation.
Why are dendritic cells considered the most potent antigen-presenting cells (APCs)?
subsets arise from different lineages (myeloid and lymphoid)
covered multiple long membrane extensions (bunch of fingers morphology)
take up antigens by phagocytosis (large eating), pinocytosis (sipping) and receptor-mediated endocytosis.
reside in tissues and capture antigens from invading pathogens and load the antigen into MHC II & I cells
migrate to lymph nodes and present these antigen to T cells and costim. T cell activation
produce cytokines to polarize immune responses
Break up flashcards above
What are the key hematopoietic growth factors for lymphopoiesis?
IL-3, IL-7, IL-2, IL-4, IL-15.
Stages of maturation in lymphoiesis are defined by what?
Surface antigen expression or cluster differentiation (CD) antigens rather than morphologic features.
Where do B cells complete their maturation?
From their progenitors within the bone marrow.
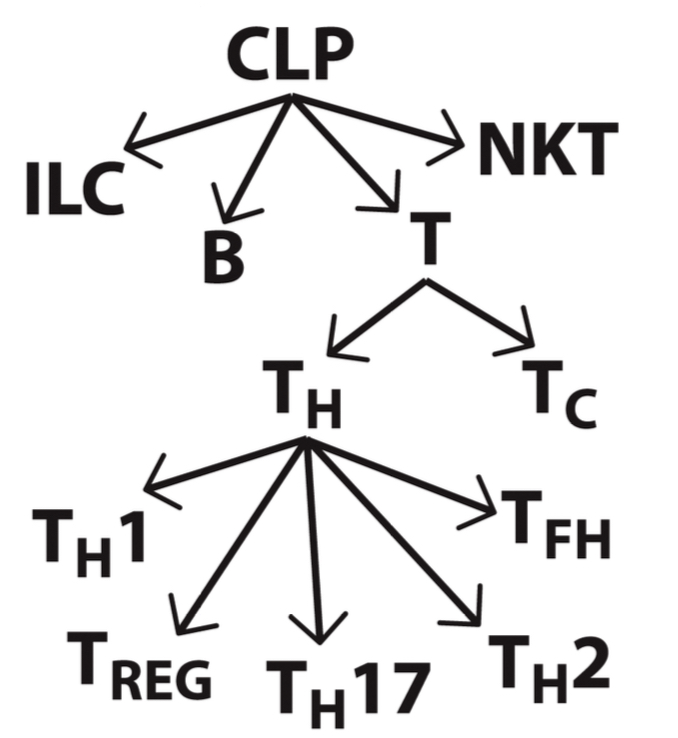
Where do T cells and NKT cells develop and mature?
They develop in the bone marrow but mature in the thymus.
Note: NK (natural killer) cells mature from their progenitors and further in the periphery and thymus (one subset).
Where do Innate lymphoid cells (ILCs) develop?
In the bone marrow then migrate to tissues.
Edit slide 32
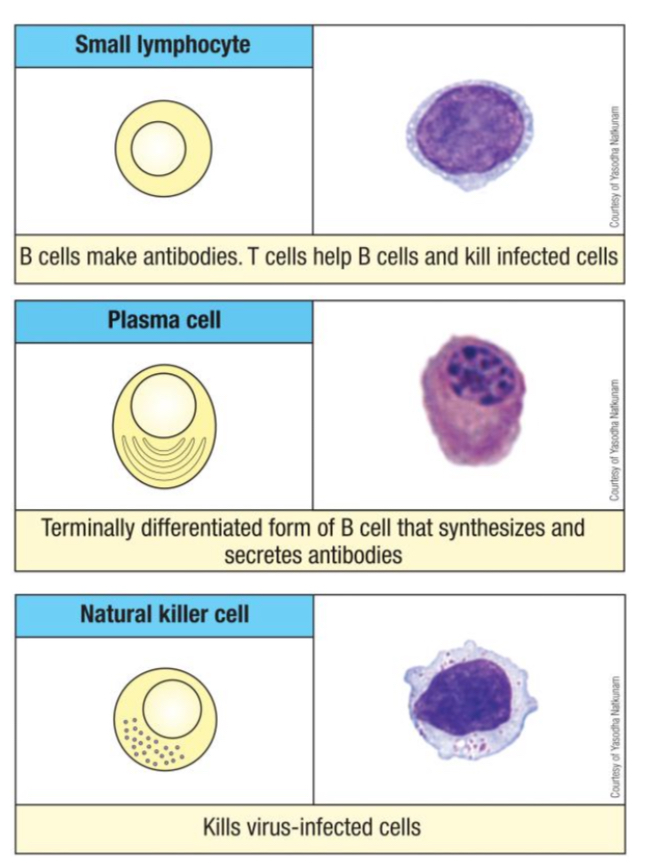
What is the typical morphology of naïve (quiescent) T and B lymphocytes?
Round nucleus (size of red cells), small rim of blue cytoplasm, approximately 9 μm in diameter.
What are the major types of T cells and their approximate percentage in lymphocytes?
T cells (CD3+), making up 70-80% of lymphocytes, further divided into
CD4+ helper T
CD8+ cytotoxic T cells
Regulatory T cells
Note: CD4:CD8 = 2:1 ratio in healthy blood
Describe the role of T cell lymphocytes before activation of the immune system?
Each exist as naïve, activated and memory cells based on experience and timing with antigen
Develop in the bone marrow, complete development in the thymus
Express an antigen receptor called the T cell receptor (TCR)
What is the primary function of CD4+ helper T cells?
They are the 'generals' of the immune response, helping to activate CD8+ T cells, B cells, macrophages, and other immune cells, and regulating immune responses by producing various cytokines.
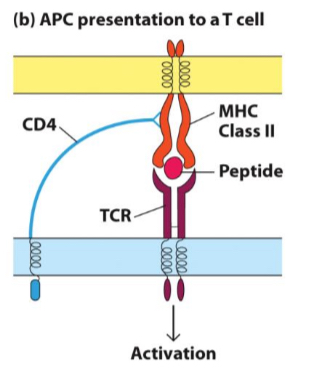
What is the role of CD8+ cytotoxic T cells?
kill virus-infected cells and cancer cells.
source of cytokines like IFN-Y
What is the role of regulatory T cells?
CD4+
Control immune responses, generally by regulating T cell reactivity
Help reduce immune response to generate rebuilding of cells/tissue (preventing autoimmune disease & strong inflammatory response persisting)
What is the main function of B cells?
10-20% (CD19+)
As naïve cells (antigen inexperienced), they express membrane-bound immunoglobulin (antibodies), and upon activation, they differentiate into plasma B cells (antibody factories) and memory B cells for memory responses. They also function as APCs in secondary immune responses.
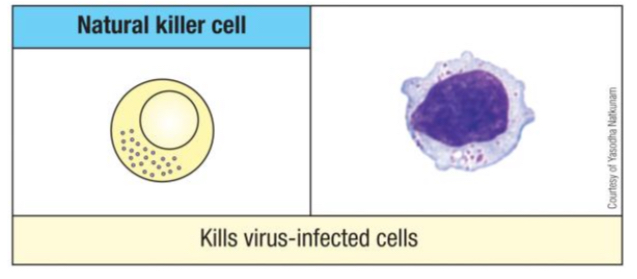
What are the unique characteristics and functions of NK cells?
They are innate-like lymphocytes that discriminate 'self' from non-self, kill target cells via granzyme/perforin and death receptors, and secrete cytokines to polarize immune responses.
What are the unique characteristics and functions of NKT cells?
Express functional T cell receptors that interact with CD1 and conserved glycolipid moieties
Develop in the bone marrow and mature in the thymus
Kill virus-infected and cancer cells
Produce pro-inflammatory cytokines to direct other immune cell
Summary of Cell Types
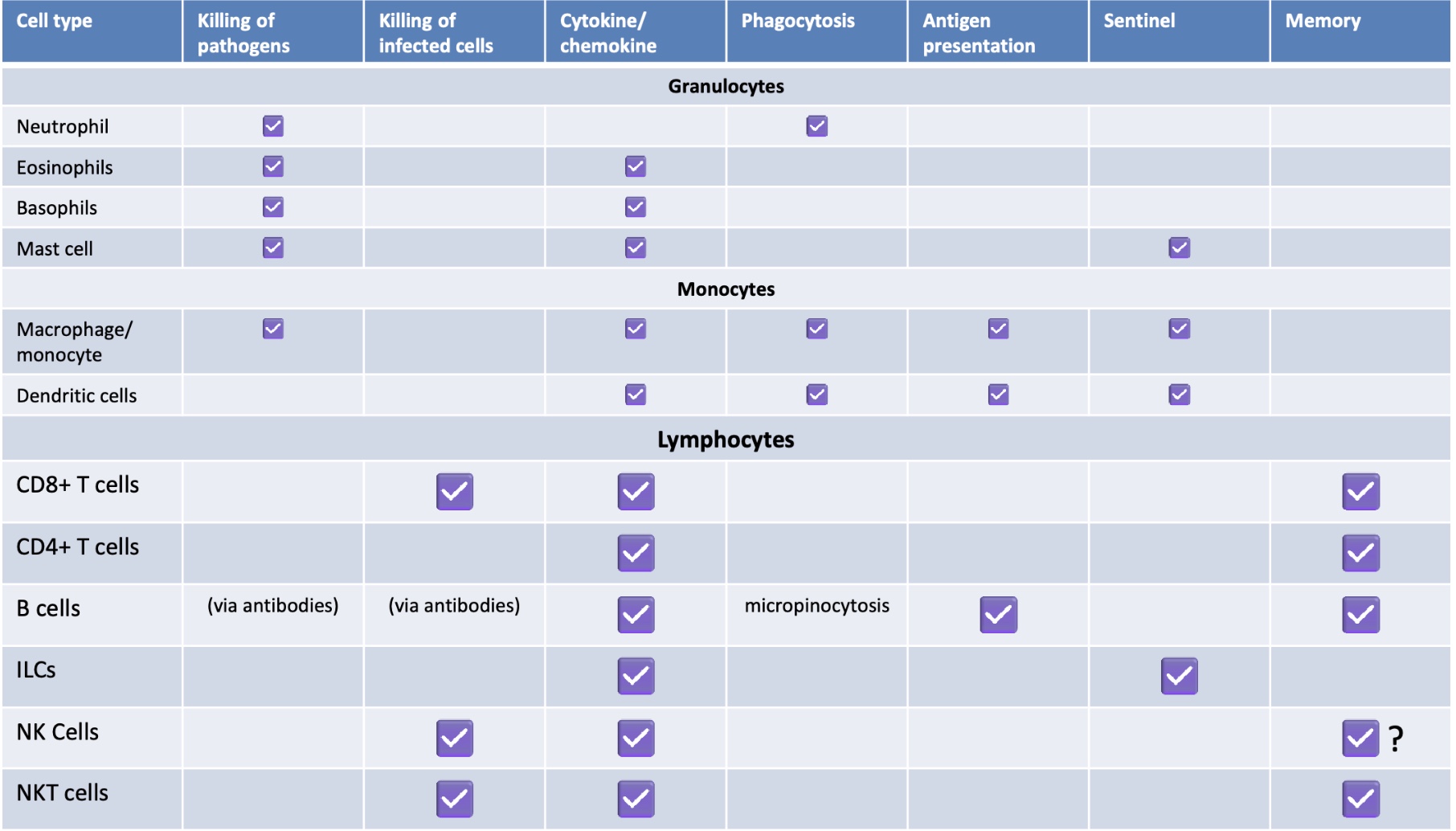
Summary of leukocyte abundance in blood
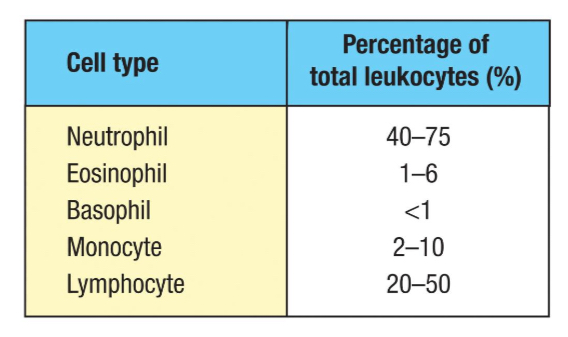
Slide 38
What are the primary lymphoid tissues and what is their function?
The thymus and bone marrow are sites of lymphocyte development and maturation.
What are secondary lymphoid tissues and what is their function?
Lymph nodes, spleen, and mucosal-associated lymphoid tissues (MALT) are sites of lymphocyte activation where antigen is trapped for interaction with mature lymphocytes.
What is the function of the thymus?
It is the site of T, NKT, and some ILC cell maturation.
What is the main function of lymph nodes?
They are sites for the generation of T cell and B cell antibody responses to specific antigens, providing a location for lymphocytes to interact with antigens and antigen-presenting cells.
How is the fluid component of blood, called lymph, returned to the bloodstream?
It is collected by lymphatic capillaries and vessels, ultimately draining via the thoracic duct into the left subclavian vein.
What do afferent lymphatic vessels do?
They bring lymph fluid containing antigen-carrying dendritic cells, particulate antigen, and a few lymphocytes from tissues to regional lymph nodes.
What are the main functions of the spleen's white pulp and red pulp?
The white pulp generates T cell and B cell responses against blood-borne antigens, while the red pulp phagocytoses defective/old red blood cells and blood-borne pathogens.