CHEM 3000
1/91
Earn XP
Description and Tags
(1) BIOCHEM INTRO (2) NUCLEIC ACIDS (3) PROTEINS AND ENZYMES
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
Biochemistry
Branch of chemistry involving chemical reactions that occur in living organisms
What are the four types of biomolecules that the human diet consist of ?
Proteins , Nucleic acids, Polysaccharides and Fats (particularly triacylglycerols)
Digestion
reduces biochemical to monomers
Monomers
Small molecules or atoms that bond together to form polymers (some monomers are: Amino acids, Nucleotides, Monosaccharides, and fatty acids
Pentose Phosphate Pathway (PPP)
(1) Glucose is broken down to make NADPH (helps with building fats and fighting harmful molecules).
→ this phase produces ribulose-5-phosphate
(2) Ribulose-5-phosphate is then changed to ribose-5-phosphate which is used to build DNA and RNA
Catabolism
The breakdown of complex molecules in living organisms to form simpler ones, together with the release of energy
Anabolism
Building up molecules
Organic Compounds
Compounds primarily made of carbon, hydrogen, and often oxygen, that are essential for life.
Saturated
Contain only carbon to carbon single bonds
Unsaturated
Contain carbon to carbon double bonds or triple bonds
Function groups
Are groups of atoms (or one atom) that impart specific chemical properties to organic compounds
Hydrocarbons
are organic compounds consisting entirely of hydrogen and carbon, which can be saturated or unsaturated
Alcohol group
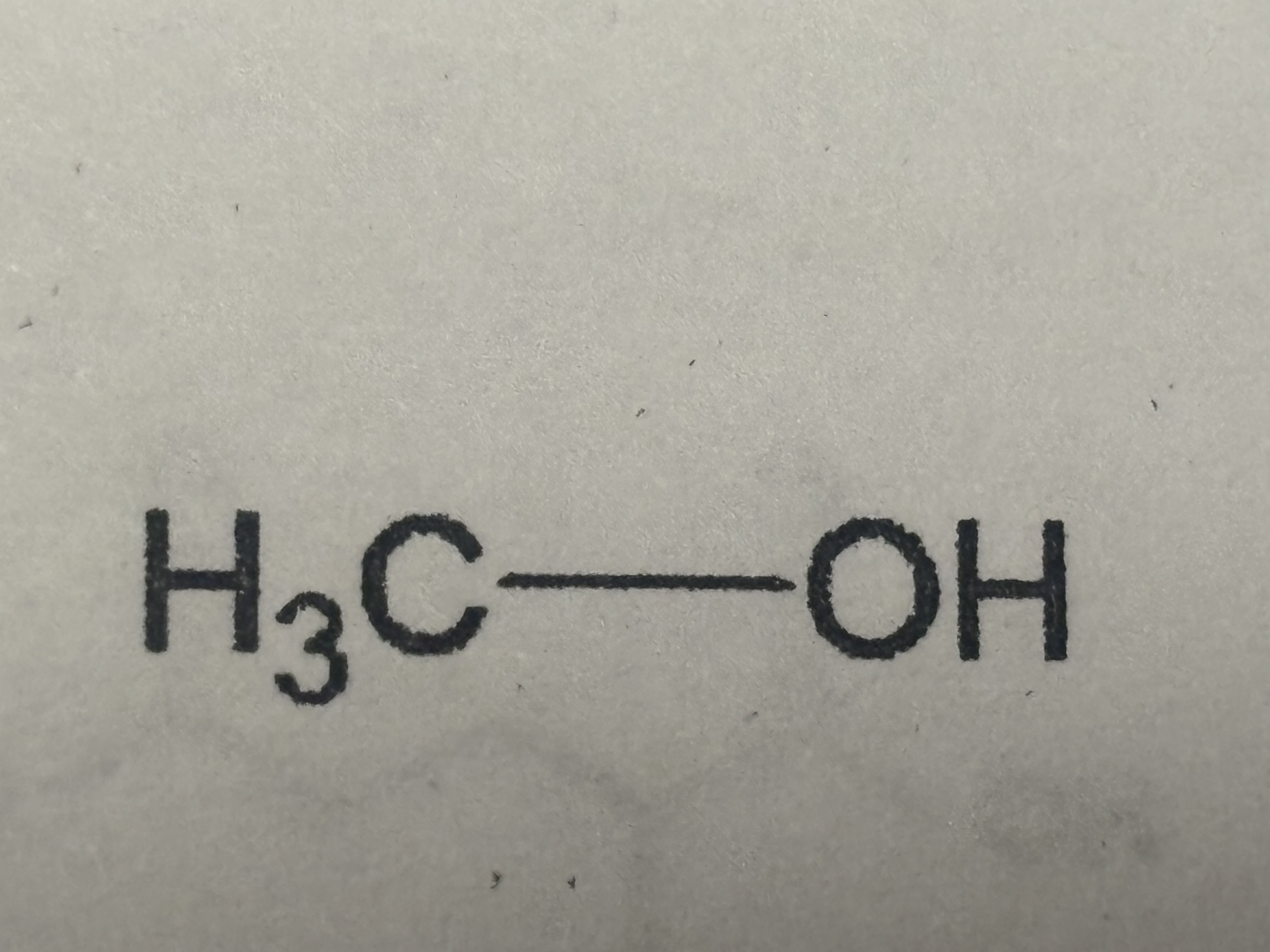
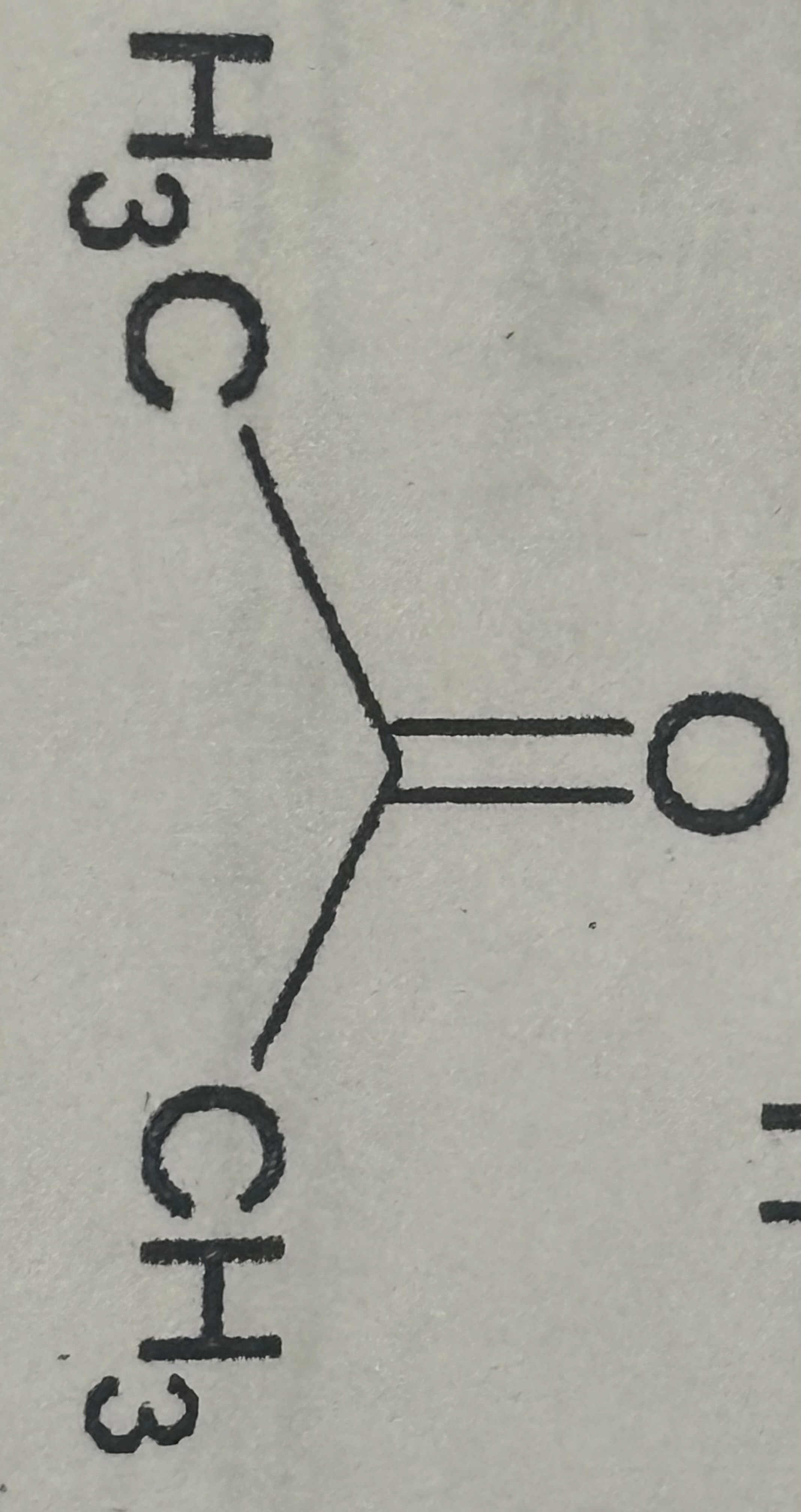
Aldehyde
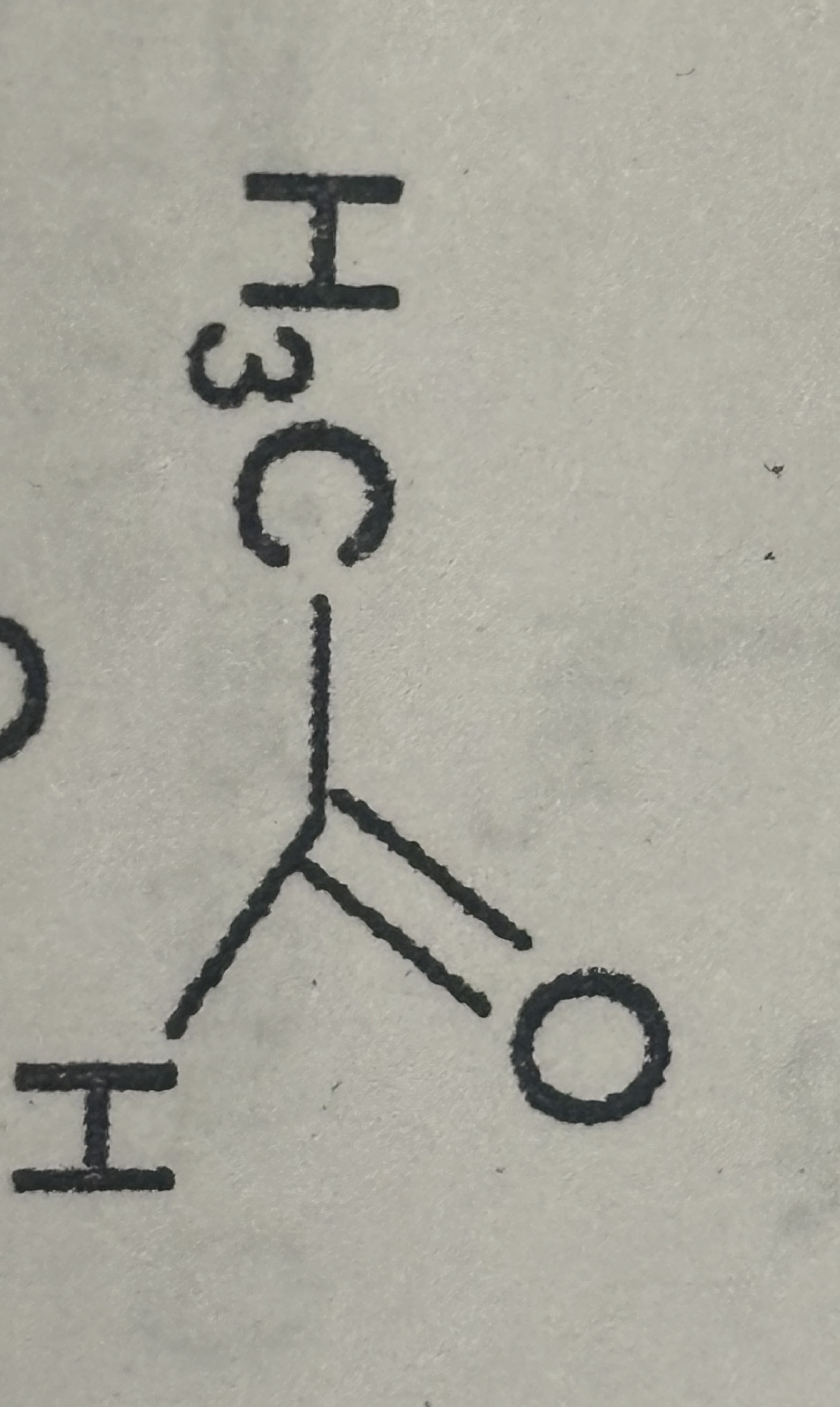
Ketone
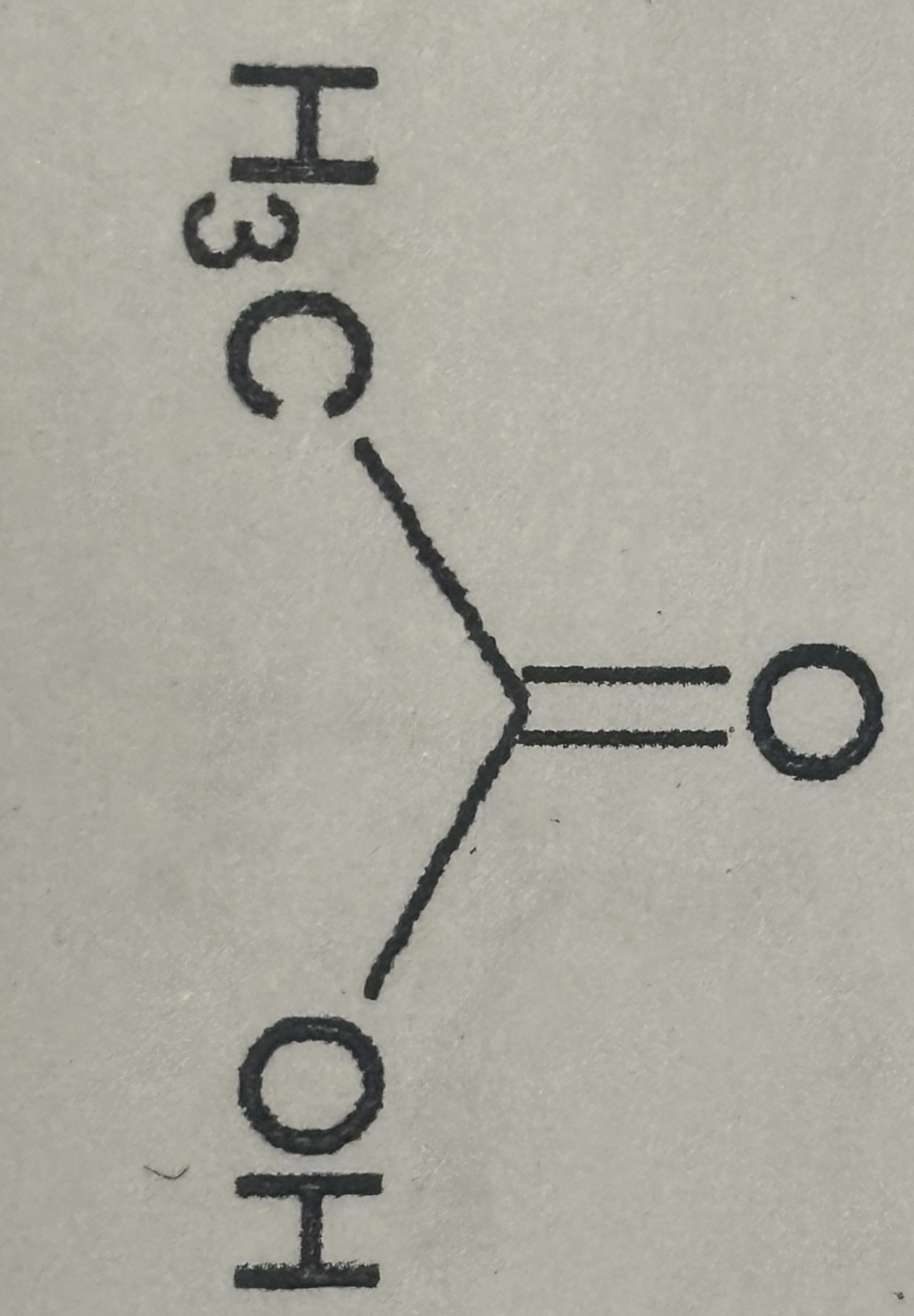
Carboxylic Acid
Ester
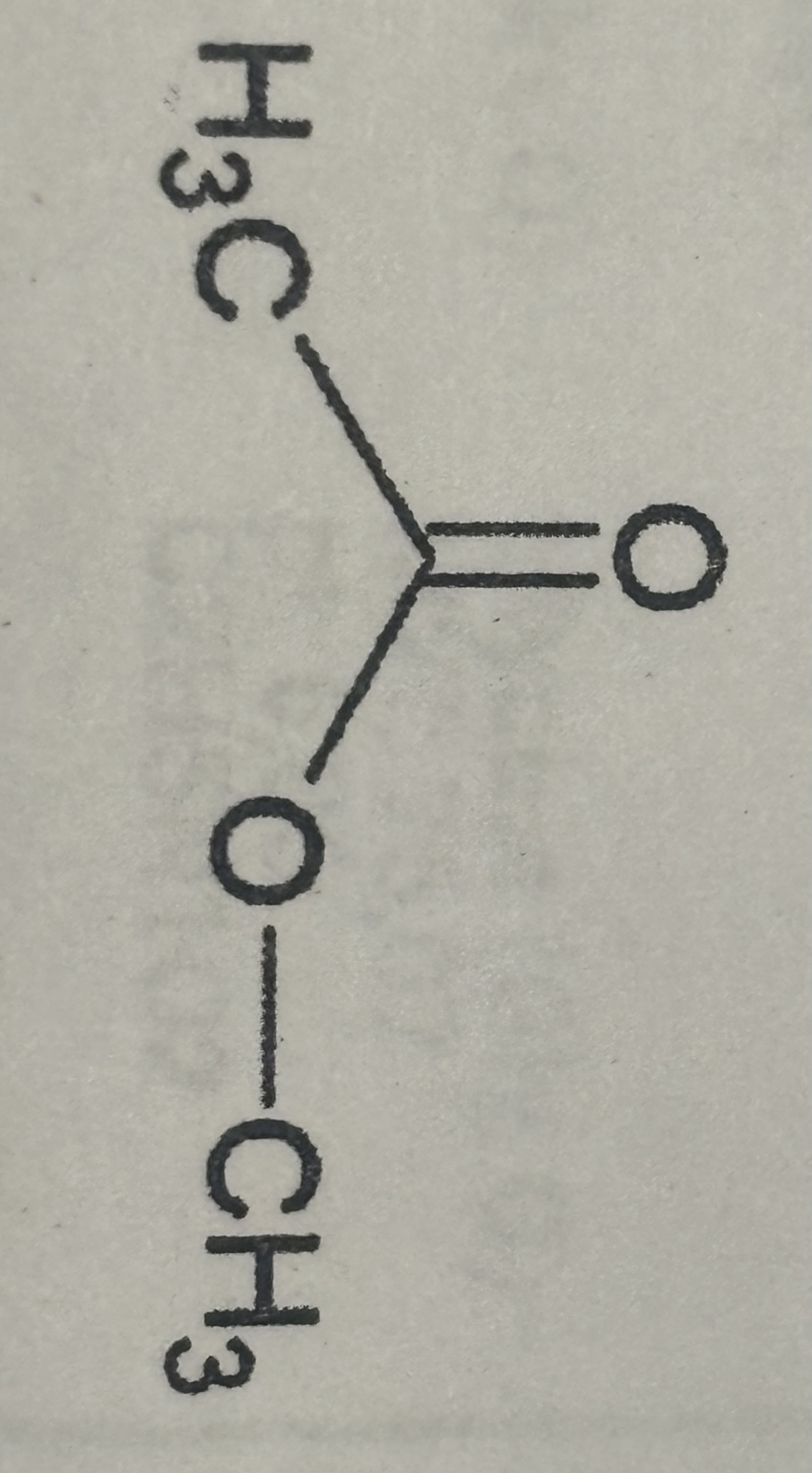
Amine
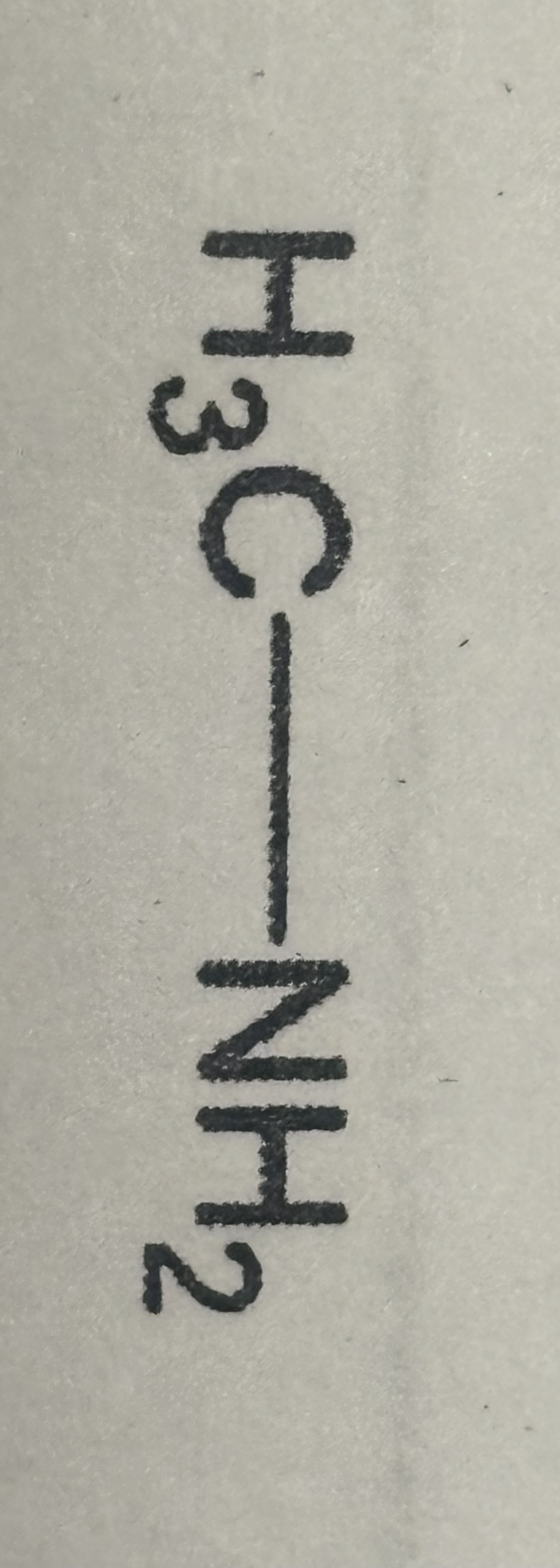
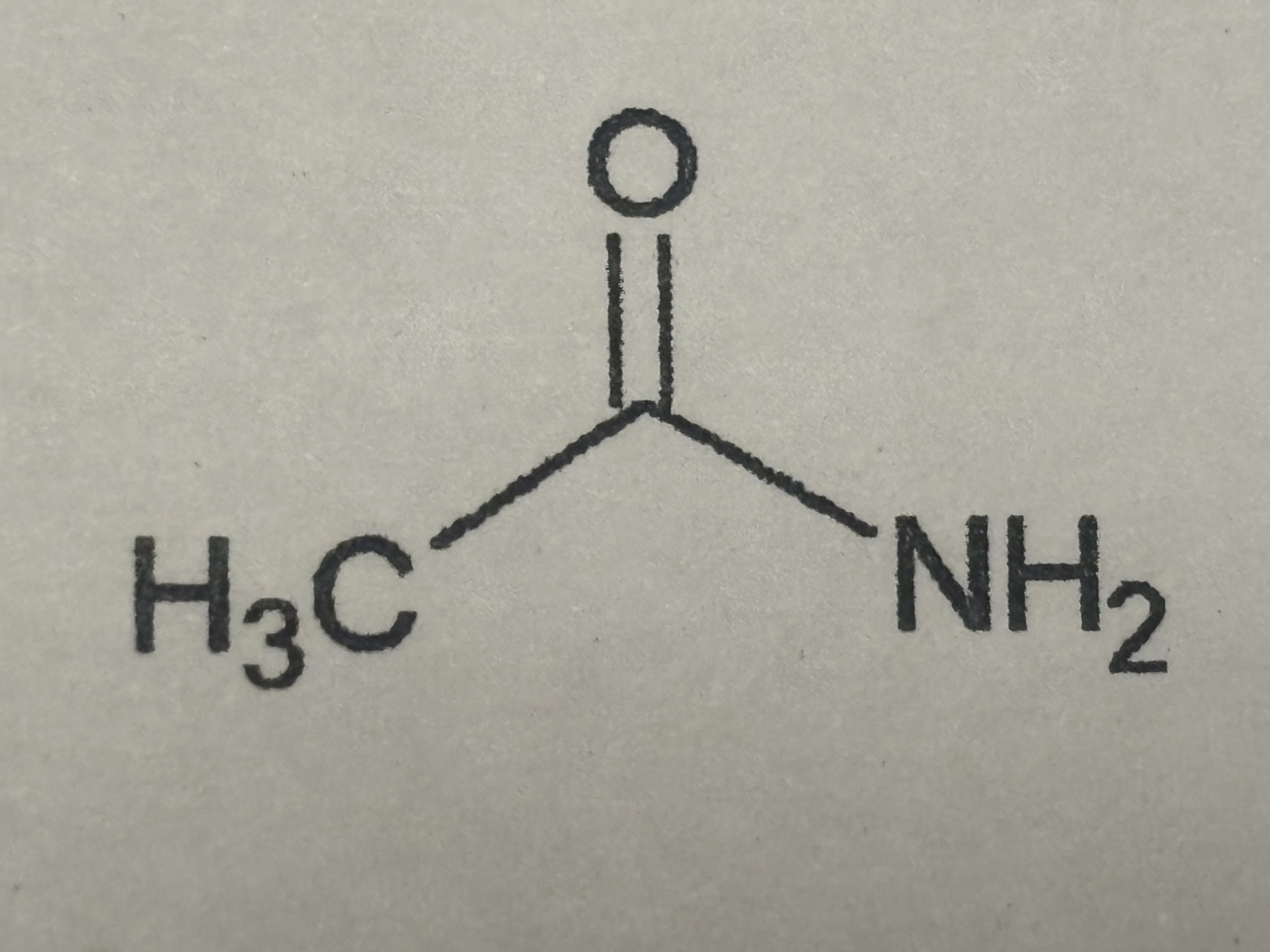
Amide
Cholesterol
waxy fat-like subtsance that your body needs for good health but in right amounts
Good Cholesterol (aka HDL)
High-density lipoprotein cholesterol if often reffered to as good because it plays a crucial role in protecting the heart. HDL travels through bloodstream, removing excess cholesterol (LDL) from the arteries and transporting it back to the liver for excretion.
Bad Cholesterol (LDL)
Low-density lipoprotein i often considered as bad because it transports cholesterol from the liver to the rest of the body having too much of this can raise the risk of heart disease and stroke.
Cholesterol Biosynthesis
Multi-step process that occurs mainly in the liver, starting from acetyl-CoA to mevalonate
(try to see the pathway in the slides)
What is Nucleic Acid composed of ?
Nucleic Acid → Nucleotides → made up od pyrimidines, purines, ribose or 2-deoxyribose and phosphate.
Structure of a nucleotide
nitrogen containing base
sugar
phosphate group
Deoxyribonucleic Acid (DNA)
Carries genetic information, composed of nucleotides (adenine, thymine, cytosine and guanine)
( DNA has an OH and H in sugar base)
Ribonucleic Acid (RNA)
A molecule that helps carry the instructions in DNA, is also made up of nucleotides (adenine, Uracil, cytosine and guanine). Plays a role in making proteins
(RNA has OH and OH in sugar base)
Pyrimidines
Single ring with two nitrogen atoms
(pyrimidine bases: cytosine and thymine)
Purine
two rings eahc with two nitrogen atoms
(purine bases: adenine and guanine)
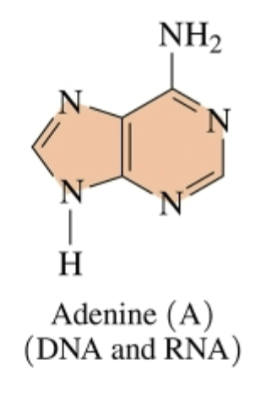
Adenine
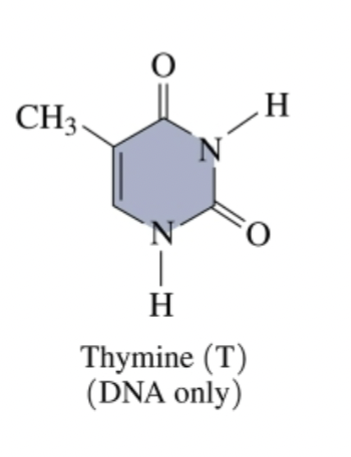
Thymine
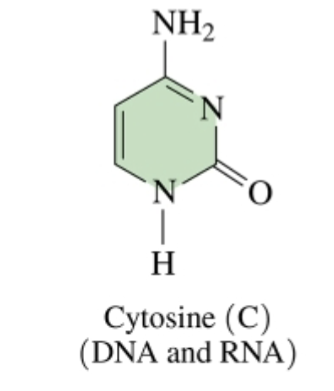
Cytosine
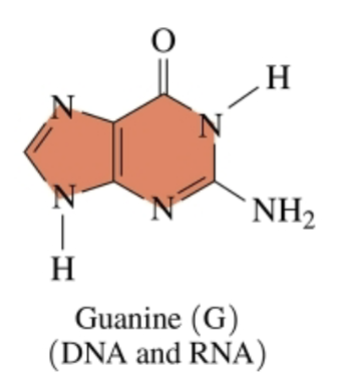
Guanine
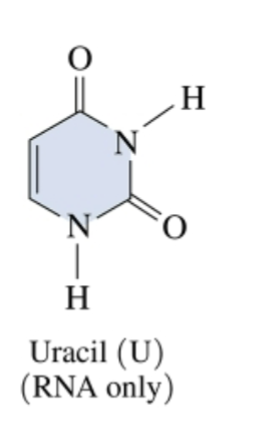
Uracil
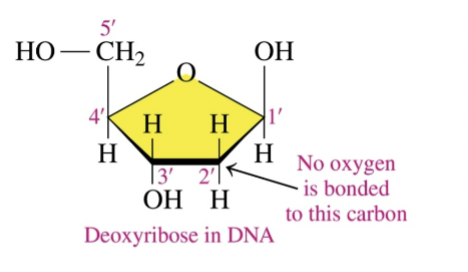
Pentose sugar in DNA
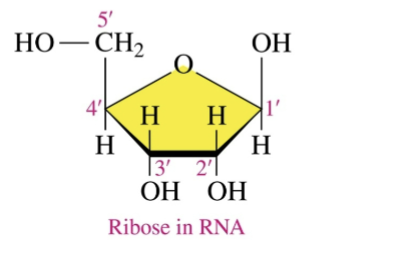
Pentose Sugars in RNA
Nucleoside
Base (nitrogenous) + Deoxyribose (or ribose)
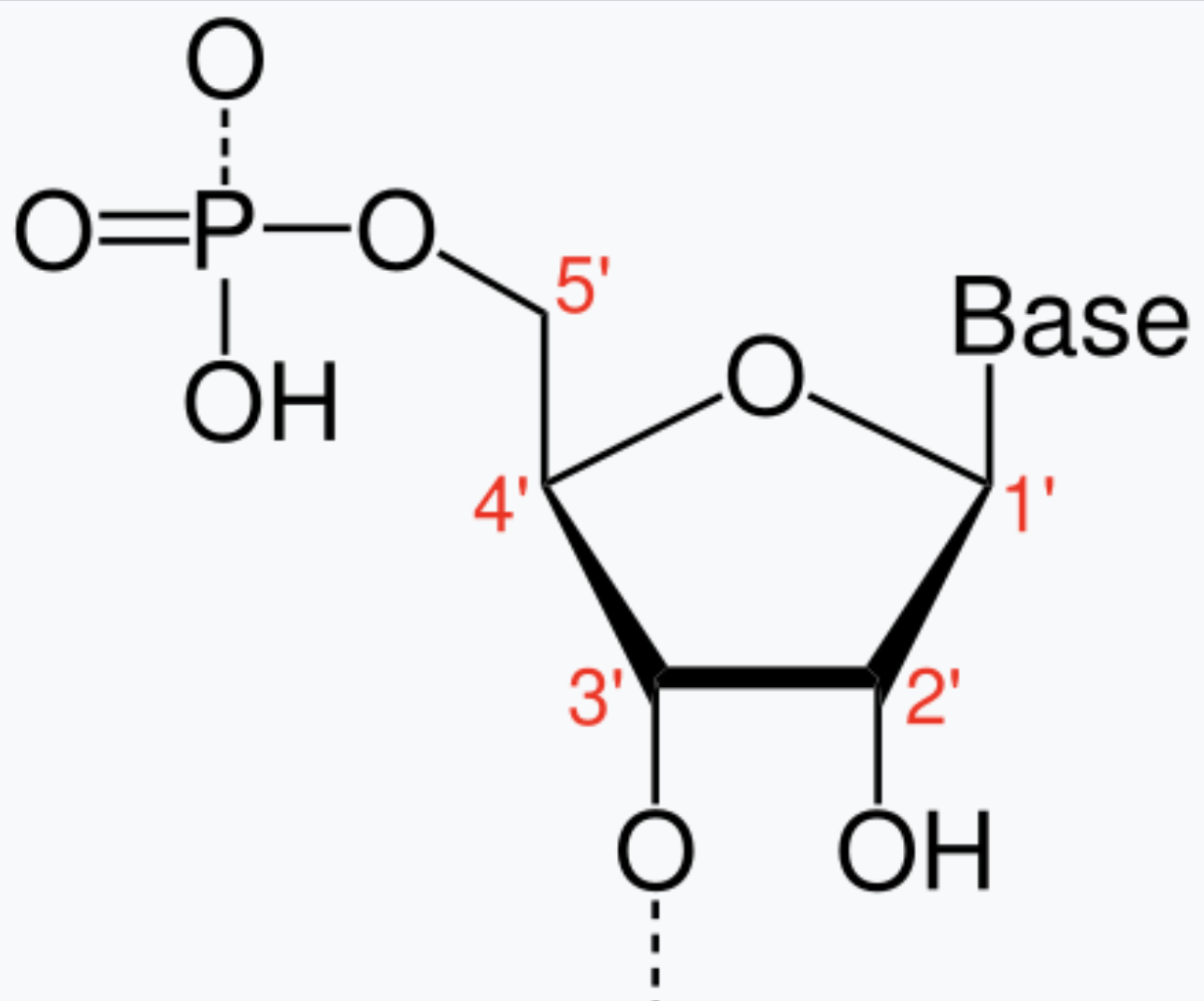
Nucleotide
Has a phosphate group attached to the C’5 carbon of OH group
( Base + deoxyribose + phosphate)
AMP, ADP and ATP
Adding a phosphate group to Adenine monophosphate forms Adenine diphosphate, then adding another phosphate group form Adenosine triphosphate (ATP)
Rules to Name Nucleosides and Nucelotides
(1) Nucleoside that contains purine ends in “osine”
(2) Nucleoside that contains a pyrimidine ends in “idine”
(3) For DNA nucleosides add “deoxy” in the beginning
(4) Nucleotides in RNA and DNA are named by adding monophosphate to the end of the nucleoside name
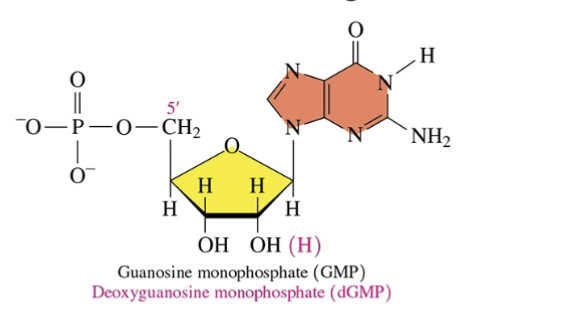
Name of this base and sugar for both DNA and RNA
DNA: Deoxyguanosine monophosphate
RNA: Guanosine monophosphate
What are nucleotides joined by ?
Phosphodiester linkage bonds
Chargaff’s rules
Number of purine molecules must equal the number of pyrimidine molecules
How many bonds for Bases A and T
Two hydrogen bonds
How many bonds for G and C
Three hydrogen bonds
Write Complementary the base for the Sequence:
5’ A G T C C A A T C 3’
3’ T C A G G T T A G 5’
( all you have to do is write the base pair to the corresponding letter, since A and T bond then T would be the complementary sequence of A )
DNA Replication process
Helicase (enzyme) - unzips the DNA by breaking the hydrogen bonds between the two strands creating a replication fork (where the DNA separates into two single strands)
Single-strand binding proteins - These proteins attach to the operates strands to prevent them from sticking back together, which keeps them open for replication.
Primase (enzyme) - This enzyme creates short RNA primers which act as starting points for the next enzyme (DNA polymerase) to begin building the new DNA strand
DNA polymerase (enzyme) - This enzyme adds new nucleotides ( A, T, C, G) to the growing strands by matching them to the complementary bases on the original strand. It builds in the 5’ to 3’ direction:
leading strand, adds nucleotides continuously
lagging strand: it works in short sections (Okazaki fragments) because DNA can only be built in one direction
DNA ligase (enzyme) - This enzyme joins the Okazaki fragments together to create a smooth continuous DNA strand.
RNA transcription
process where a cell makes a copy of gene’s DNA to RNA
An enzyme called RNA polymerase binds to the DNA at a specific spot called (promoter).
RNA polymerase moves along the DNA reading the sequence and building a complementary RNA strand ( using A, C, U and G)
Once the RNA strand is complete it detaches and the DNA rewinds back
Messenger RNA
mRNA carries genetic information from DNA to the ribosomes
( 5% of mRNA exists)
Transfer RNA
tRNA translates genetic information in mRNA into the amino acid sequence for the protein.
Has an L-shape and an acceptor stem at the 3’ end with the nucleotide sequence ACC
An enzyme attaches to the sequence and binds to the amino acid using an enzyme
also contains an anticodon which is a series of bases that complements the bases that match up with mRNA bases to ensure the right amino acid is added to the protein.
( 15% of this tRNA exist)
Ribosomal RNA
rRNA most abundant type, it is combines with proteins to form ribosomes.
RNA and Protein synthesis
How cells make proteins from DNA instructions. it happens in two main steps: transcription and tranbslation
RNA protein synthesis process
Transcription (making RNA): Dna acts like a recipe book for proteins. An enzyme called RNA polymerase makes a copy from DNA to mRNA, this mRNA carries the message out of the nucleus to a ribosome ( think of it like a protein factory)
Translation (making protein): ribosomes read the mRNA instructions like a person reading a recipe. tRNA then brings out the right amino acids to the ribosomes, amino acids are linked together to form a protein (like putting beads on a string). The proetin then folds into its correct shape. E
Exons
The coding regions of a gene in mRNA remain after splicing (the process of removing introns) and are used to make proteins.
Introns
Non-coding regions of a gene in pre-mRNA that are removed during splicing and do not contribute to the final protein
mRNA processing: exons and introns
process explains how pre-mRNA is processed before it becomes mature mRNA which is used to make a protein
Pre-mRNA: contains both exons and introns
Processing (splicing): introns are removed because they don’t code
Mature mRNA: only the axons remain and this final version is sent out to the nucleus to be used for protein production
( think of editing a rough draft and removing unnecessary parts which are the introns here)
Regulation of transcription
Controls when and how much RNA is made from a gene. This ensures proteins are made only when needed.
(some controls are:
Transcription factors - proteins that turn genes on or off
Promoters and enhancers - DNA regions that control transcription strength
Repressors - proteins that block transcription
Epigenetic changes - modifications like DNA methylation that silence or activate genes
Genetic code and protein synthesis
Genetic code ensures that the right proteins are made. Protein synthesis creates enzymes hormones and structural proteins needed for body functions (process transcription and translation)
Protein synthesis Activation, initiation, elongation and termination
Activation: Before Translation starts, tRNA molecules must be activated. tRNA activation occurs when an enzyme attaches the correct amino acid to a tRNA, this ensures each tRNA carries the right amino acid to the ribosome
Initiation: Ribosome attaches to the mRNA at the start codon AUG, the first tRNA carrying Methionine binds to the start codon. The ribosome assembles and translation begins.
Chain elongation: The ribosome moves along the mRNA reading codons, and the tRNA molecule brings amino acids which are linked together one by one. A peptide chain (protein) grows as the ribosome continues translating.
Termination: The ribosome keeps reading until it reaches a stop codon (UAA, UAG, or UGA). since no tRNA matches the stop codon a release factor binds instead the ribosome lets go of the mRNA and the new protein folds it into its correct shape and goes to the cell to do its job.
Transcription Factors
They are proteins that bind to the promoter region of a gene to help RNA polymerase attach to DNA and start transcription, controlling when and how genes are turned on or off
Amino Acids
Molecular building blocks of proteins, they have a central carbon atom bonded to two functional groups: an ammonium group ( — NH +3 ) a carboxylate group ( — COO - ), and an R group ( CH3 )
Zwitterions
A molecule that has both positive and negative charge at the same time but is overall neutral.
for example, amino acids are zwitterions IN WATER because the NH group is positively charged and the carboxyl group is negative so they cancel out and are neutral.
L-amino acids
have the - NH3 + on the ;eft side when drawn in a fisher projection
D-amino acids
The - NH3+ is in the right side in a fischer projection
Isoelectric point (pl)
The pH at which an amino acid or protein has a neutral overall charge with equal positive and negative charges balancing out
ionized forms of amino acids
At different pH levels, amino acids can gain or lose charges on their amino and carboxyl groups, leading to different ionized forms
low PH (acidic) - amino groups stay positively charged but the carboxyl becomes neutral
At neutral Ph (7) - the amino group is positively charged and the carboxyl is negatively charged ( this results in zwitterions)
At High pH (basic conditions) - amino becomes neutral and the carboxyl group stays negatively charged.
Enzyme
are proteins that act like catalyst, speeding up chemical reactions in the body without being used up in the process. They work by lowering the activation energy needed for a reaction to occur, making reactions happen more easily, Each enzye is specific to a particular reaction or substrate (the molecule it acts on)
Optimum pH
the pH at which an enzyme works most efficiently and produces fastest reaction rate. Different enzymes have optimum pH levels depending on their environment.
Peptide bond
A chemical bond that forms between the carboxyl group ( —COOH) of one amino acid and the amino group of anther amino (-NH₂) of another amino acid, releasing a molecule of water and linking two amino acids in a chain this bind forms the backbone of proteins
Quaternary structures
The structure of a protein is when two or more polypeptide chains ( aka protein subunits) come together to form one functional protein
Tertiary structure
The structure of a protein is its 3D shape, formed when the polypeptide chain Folds and Twist due to different interactions between amino acids
Naming peptides
Identify the amino acid in the chain - peptides are made of amino acids linked by peptide bonds.
start naming from the N-Terminus (left side) - The first amino acid on the N-terminal/left side keeps its full name. All the following amino acids end in “yl” except the last one
Keep the last amino acid’s full name - The last amino acid (C-terminal) keeps its full name WITHOUT the “yl”
example: naming a tripeptide
glycine → Glycyl | alanine → Alanyl | serine → serine (keeps full name)
= Glycylalanylserine
Protein Hydrolysis
Process of breaking down a protein into smaller peptides or amino acids by adding water and using enzymes or acids
Enzyme specificity
The ability of an enzyme to act on only one specific substrate or type of reaction due to the unique shape if its active site. (think of a lock and key)
Enzyme-Catalyzed Reaction / Enzyme substrate Complex
The enzyme binds with the substrate (the molecule it works on) to form an enzyme-substrate (ES) complex.
The enzyme creates an easier way for the reaction to happen using less energy
Inside the active site, amino acids help transform the substrate into the product.
The enzyme-product (EP) complex is formed, and the product is released. The enzyme can repeat the process.
Phosphorylation
The process of adding a phosphate group (PO₄²⁻) to a molecule, usually a protein, by an enzyme called a kinase. This process modifies the protein’s function, often by activating or deactivating it, and plays a key role in regulating cellular activities like signal transduction and metabolism. (its like a switch that turns proteins on or odd to control cellular activities)
Enzyme regulation
The control of enzyme activity to ensure the reactions occur at the right time and speed in the body, It helps maintain metabolic process.
Allosteric enzymes
enzymes that can be activated or inhibited by binding molecules at allosteric sites (not active sites)
activator: increases enzyme activity
(both activator and inhibitor change the shape of the enzyme)
Inhibitor: decreases enzyme activity
feedback control
The end product of a metabolic pathway can inhibit an enzyme earlier in the pathway to regulate the reaction and prevent excess product
Covalent Modifications
Phosphorylation or other modifications (like adding a phosphate group) can activate or deactivate enzymes
Inhibitors
Molecules that cause a loss of catalytic activity. Prevents substrate from fitting into the active sites can be classified as either reversible inhibitor or irreversible inhibitors
Enzyme Inhibitor
a molecule that slows down or stops the activity of an enzyme by binding to it and preventing it from catalyzing a reaction
Reversible inhibition
When an enzyme inhibitor binds to an enzyme and stops its activity but the inhibitor can be removed or disassociated from the enzyme allowing the enzyme to work again.
Competitive inhibitors
The inhibitor competes with the substrates for the active site. The inhibitor may look like the substrate preventing the real one from attaching. Because both the inhibitor and substrates compete for the same spot the reaction slows down. If you add more substrate it can outcompete the inhibitor allowing the enzyme to work
Antimetabolites
They are medicine that mimic natural molecules (metabolites) the body uses in biochemical reactions. They block or interfere with the normal process of metabolism. Some antibiotics are competitive inhibitors that block bacterial enzymes by mimicking the substrates, the antimetabolite blocks the enzyme preventing bacteria from growing.
Non Competitive Inhibitors
The inhibitor binds to a different site on the enzyme, changing its shape. The inhibitor can be removed allowing the enzyme to return to its normal function
Uncompetitive inhibition
The inhibitor binds only to the enzyme-substrate complex preventing the reaction like the rest this can be reversed by removing the inhibitor
Irreversible inhibition
occurs when an inhibitor permanently binds to an enzyme destroying or blocking its ability to function. When this happens the enzyme cannot be reused and the cell must produce new enzymes to replace it. ( often used to block harmful enzymes in certain types of cancer)
Simple Enzyme
Active enzyme that consist of ONE protein
Cofactor
a non-protein molecule that helps an enzyme work properly it can be an inorganic ion like Zinc (ZN2-) or iron (Fe2+) some enzymes need cofactors to be active
Coenzyme
A type of cofactor that is a small organic molecule (often derived from vitamin) coenzymes help enzymes by transferrign chemical groups between molecule during reactions
Metal ions as cofactors
Some enzymes need metal ions to function properly. These metal ions act as cofactors helping the enzyme carryout its job