Y8 Separation techniques keywords
0.0(0)
Card Sorting
1/20
There's no tags or description
Looks like no tags are added yet.
Last updated 3:40 PM on 2/26/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
1
New cards
Mixture
A substance comprised of two or more substances combined but NOT chemically bonded/joined.
2
New cards
Solvent
A liquid or gas that dissolves a solute.
3
New cards
Solute
The substance that dissolves in a solution (often a solid).
4
New cards
Solution
A mixture of a solute and a solvent.
5
New cards
Soluble
Can dissolve.
6
New cards
Insoluble
Cannot dissolve.
7
New cards
Saturated solution
A solution in which no more solute can dissolve at a particular temperature.
8
New cards
Solubility
A measure of how much solute can dissolve per 100g solvent.
9
New cards
Pure
A substance is Pure if it has no other substances mixed with it.
10
New cards
Evaporation
When a liquid turns into a gas.
11
New cards
Condensation
When a gas turns into a liquid.
12
New cards
Distillation
A separation technique used to separate a mixture of liquids by their boiling points.
13
New cards
Miscible
Capable of being mixed.
14
New cards
Filtration
A separation technique used to separate an insoluble solid from a solution.
15
New cards
Residue
The solid left on the filter after filtration.
16
New cards
Filtrate
The liquid that passes through the filter and is collected.
17
New cards
Element
A substance made up of one type of atom.
18
New cards
Compound
A substance made of two or more elements chemically bonded/joined together.
19
New cards
What type of mixture can be separated by using filtration?
A solvent and an INSOLUBLE solid (e.g. water and sand).
20
New cards
What is the formula for calculating Rf value?
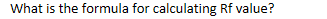
Rf = distance moved by spot ÷ distance moved by solvent front

21
New cards
If a spot/dye moves very far up the paper chromatogram what does this mean about its solubility?
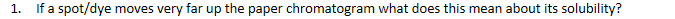
The further the spot moves up the MORE soluble it is