Biochem Final
0.0(0)
0.0(0)
Card Sorting
1/137
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
138 Terms
1
New cards
What is an aquaporin and what does it do?
prevents proton hopping and transports water across membranes, is a glycoprotein and is a homotetramer
2
New cards
carbohydrates
carbon-based molecules rich in hydroxyl groups (CH2O)6
* monosaccharides: aldehydes or ketones with 2+ OH groups, exist in many different isomeric forms
* disaccharides: 2 monosaccharides
* polysaccharides: oligosaccharides 2+ linked by O-glycosidic bonds (OH groups like Ser and Thr), other polysaccharides can have N-links (N groups like Asn)
* monosaccharides: aldehydes or ketones with 2+ OH groups, exist in many different isomeric forms
* disaccharides: 2 monosaccharides
* polysaccharides: oligosaccharides 2+ linked by O-glycosidic bonds (OH groups like Ser and Thr), other polysaccharides can have N-links (N groups like Asn)
3
New cards
aldoses
carbohydrate monosaccharide with an aldehyde group
4
New cards
ketoses
carbohydrate monosaccharide with a ketone group
5
New cards
polysaccharide bonding
* 1,4 glycosidic bonds
* 1,6 glycosidic bonds form branches
* 1,6 glycosidic bonds form branches
6
New cards
isomers
have the same molecular formula but different structures
7
New cards
constitutional isomers
differ in order of attachment of atoms
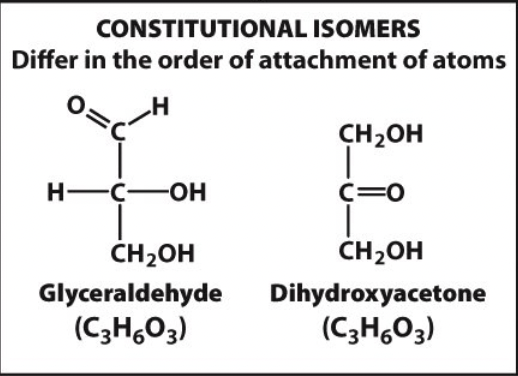
8
New cards
stereoisomers
atoms connected in the same order but differ in spatial arrangement, includes: enantiomers and diastereomers → which include epimers and anomers
9
New cards
enantiomers
nonsuperimposable mirror images
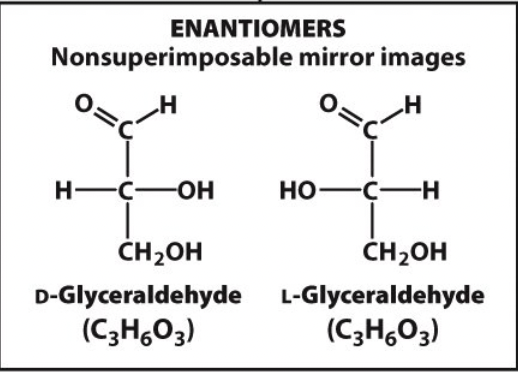
10
New cards
diastereomers
isomers that are not mirror images
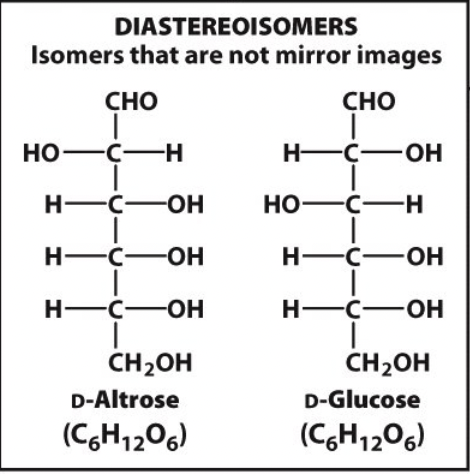
11
New cards
epimers
diastereomer that differs at one of several asymmetric carbon atoms
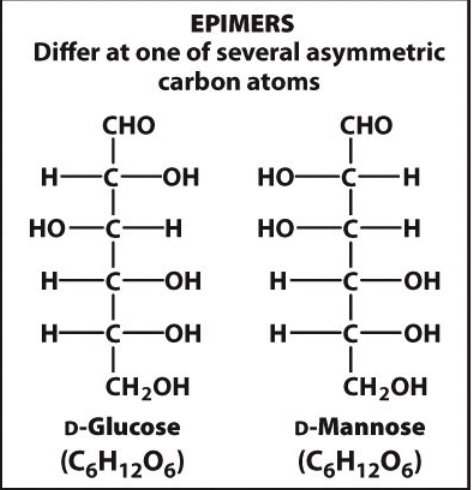
12
New cards
anomers
isomers that differ at a new asymmetric carbon atom formed on ring closure
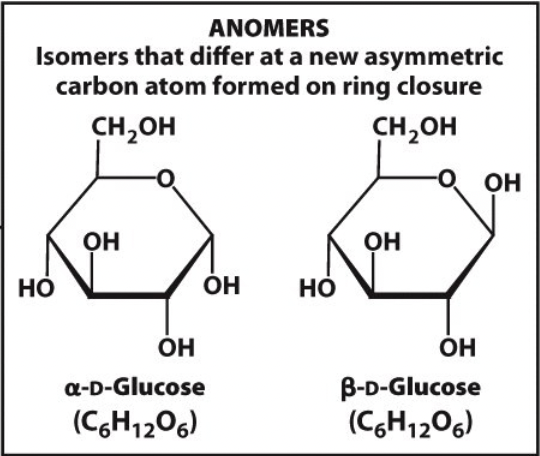
13
New cards
starch
made of amylopectin (branched) and amylose (unbranched) and is water insoluble due to layered semi-crystalline form, alpha linkages
14
New cards
cellulose
straight chains that form string fibrils, strong and durable, made of beta 1,4 linkages
15
New cards
glycogen
alpha glucose subunits, highly branched (1,4 and 1,6 linkages)
16
New cards
glycoproteins
carbohydrates attached to proteins, largest component by weight is protein
17
New cards
proteoglycans
protein attached to glycosaminoglycan, important for structural roles (cartilage), composed of repeating disaccharide with sulfate modification
18
New cards
mucins/mucoproteins
predominantly carbohydrate, pretein attached to carbohydrate N-acetylgalactosamine
19
New cards
phosphorylation in carbohydrates
excessive phosphorylation disrupts branching pattern and structure of glycogen, can lead to neurodegeneration
20
New cards
starch degredation
outermost layer becomes water soluble
21
New cards
lipids
non-polymer, water insoluble, critical for energy storage and signaling
* free fatty acids
* triglycerols
* phospholipids
* glycolipids
* steroids
* free fatty acids
* triglycerols
* phospholipids
* glycolipids
* steroids
22
New cards
fatty acids
long hydrocarbon chain, can be saturated (solid) or unsaturated (liquid, double bond),
18: 2n-6 means 18 carbons, 2 double bonds, double bond 6 from omega (CH3) end
18: 2n-6 means 18 carbons, 2 double bonds, double bond 6 from omega (CH3) end
23
New cards
Triglyceride
esterified fatty acids, storage form of fatty acids
24
New cards
glycerophospholipids
They have four components: fatty acids (2 or \n more), a glycerol platform , a phosphate, and \n an alcohol
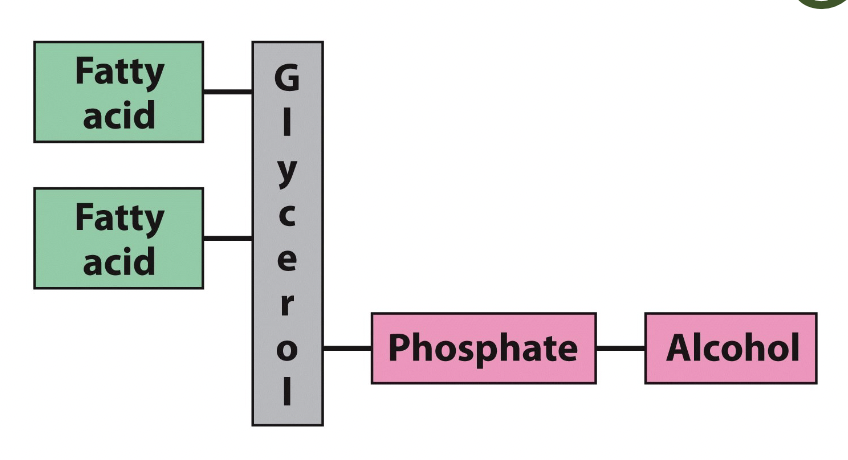
25
New cards
glycolipids
carbohydrate-containing lipids, important for cell signaling
26
New cards
cholesterol
helps maintain membrane fluidity, less van der waals, worse packing, increases fluidity
27
New cards
lipid-bilayer
2 molecules thick of phospholipids, semi-permeable, creates 2 environments
28
New cards
passive transport
mediated by ionophores
29
New cards
ion channels
highly selective for an ion, removes water in interior wall through selectivity filter (S4 is Arg rich +), are gated
30
New cards
Porins
transport ions and nonpolar solutes, transport bigger molecules than ion channels, beta barrel
31
New cards
Transporters
alternate between 2 conformations to move substances from one side to another
32
New cards
secondary transporters
* Symporters power the transport of a molecule against its concentration \n gradient by coupling the movement to the movement of another molecule \n down its concentration gradient, with both molecules moving in the same \n direction
* Antiporters also use one concentration gradient to power the formation of \n another, but the molecules move in opposite directions
* Antiporters also use one concentration gradient to power the formation of \n another, but the molecules move in opposite directions
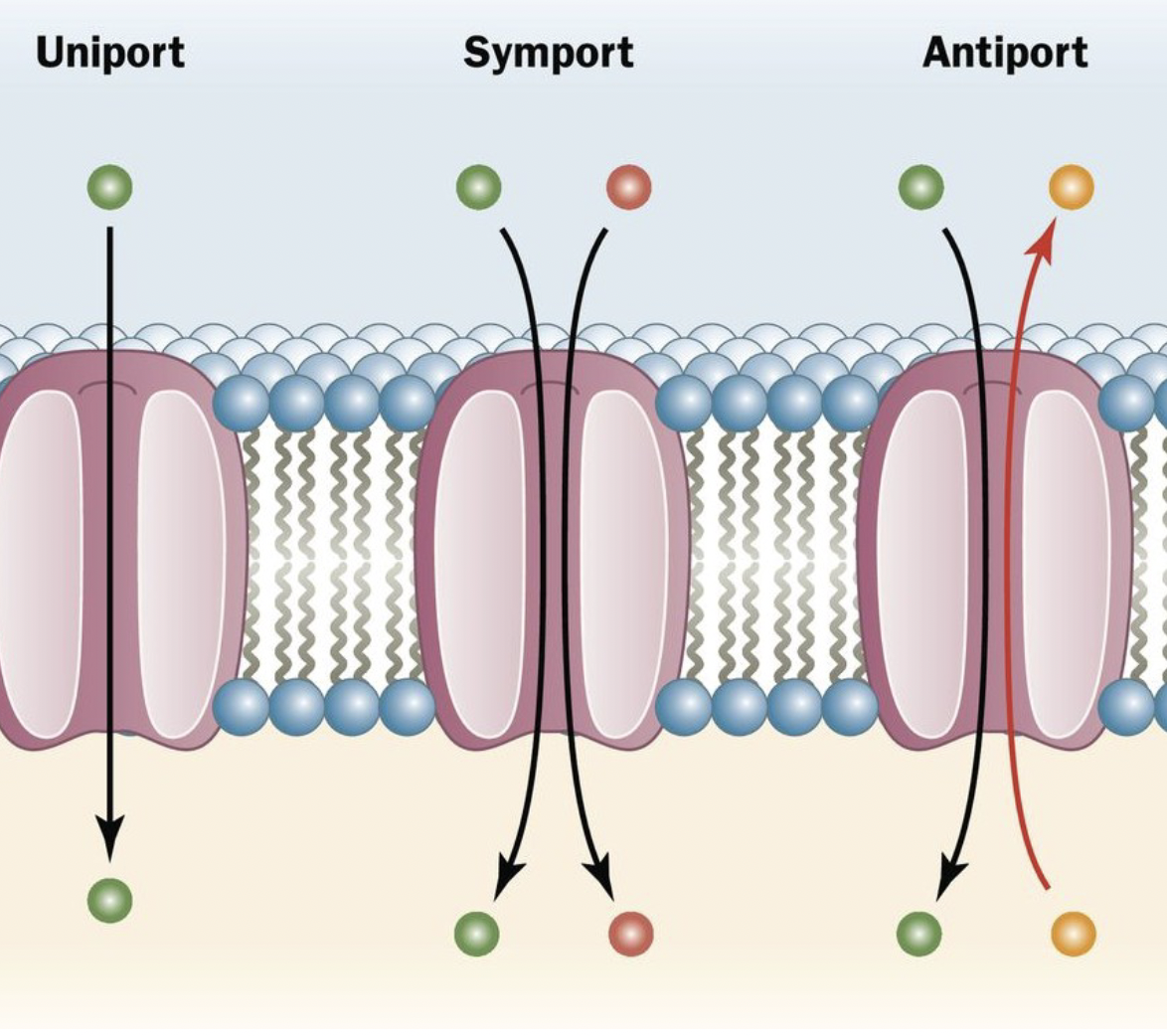
33
New cards
ABC transporters
pump ions, sugars, amino acids, polar and non polar substances (outward facing to inward)
34
New cards
enzymes
increase reaction rates by lowering activation energy, usually proteins but can be RNA, provide specific environment and reaction takes place in confined space called the active site
35
New cards
induced fit model
substrate and enzyme are not exact fit, adapts shape
36
New cards
lock and key model
enzyme and substrate are exact fit (not always true)
37
New cards
substrate specificity
geometric specificity and electronic specificity
38
New cards
how enzymes work
* regenerate
* lower activation barrier
* do not change equilibrium
* activation E reflects rate of reaction
* reaction reaches equilibrium faster
* lower activation barrier
* do not change equilibrium
* activation E reflects rate of reaction
* reaction reaches equilibrium faster
39
New cards
enzyme reactions
\
* Enzymes function as catalysts \n by stabilizing the transition
state
* Active site matches the shape of the \n transition state leading to product
* Active site possesses functional \n groups that interact more strongly \n with the transition state structure \n than with the substrate
* Enzymes function as catalysts \n by stabilizing the transition
state
* Active site matches the shape of the \n transition state leading to product
* Active site possesses functional \n groups that interact more strongly \n with the transition state structure \n than with the substrate
40
New cards
how is activation energy lowered
the binding of the substrate to the enzyme releases energy that is later used to lower the activation energy
41
New cards
in transition state what interactions are optimized?
weak interaction between enzyme and substrate
42
New cards
cofactors
enhance the range of enzymatic reactions (metal ions and coenzymes)
43
New cards
active form
holoenzyme
44
New cards
inactive form
apoenzyme
45
New cards
enzyme kinetics importance
* insight to reaction mechanisms
* might need to know how quickly a reaction happens
* regulation of reaction mechanisms
* gives foundational understanding of living organisms and gives us the ability to modulate
* might need to know how quickly a reaction happens
* regulation of reaction mechanisms
* gives foundational understanding of living organisms and gives us the ability to modulate
46
New cards
Vmax
max velocity at enzyme saturation
47
New cards
Vo
initial velocity a \[S\]
48
New cards
Km
michaelis-menton constant, describes substrate concentration needed to achieve half of Vmax (specific to substrate, temp, and pH)
49
New cards
Kcat
turnover number (number of reactions for unit time)
50
New cards
kcat/km
specificity number (measure of enzyme efficiency)
51
New cards
catalytic perfection reached at
10^8 to 10^9 M-1\*s-1
52
New cards
michaelis menton equation
Vo = (vmax \[S\]) / (Km + \[S\])
53
New cards
kinetic perfection
catalytic velocity is restricted only by the rate at \n which they encounter substrate in solution
54
New cards
types of inhibition
* irreversible
* reversible
* competitive
* uncompetitive
* non-competitive
* reversible
* competitive
* uncompetitive
* non-competitive
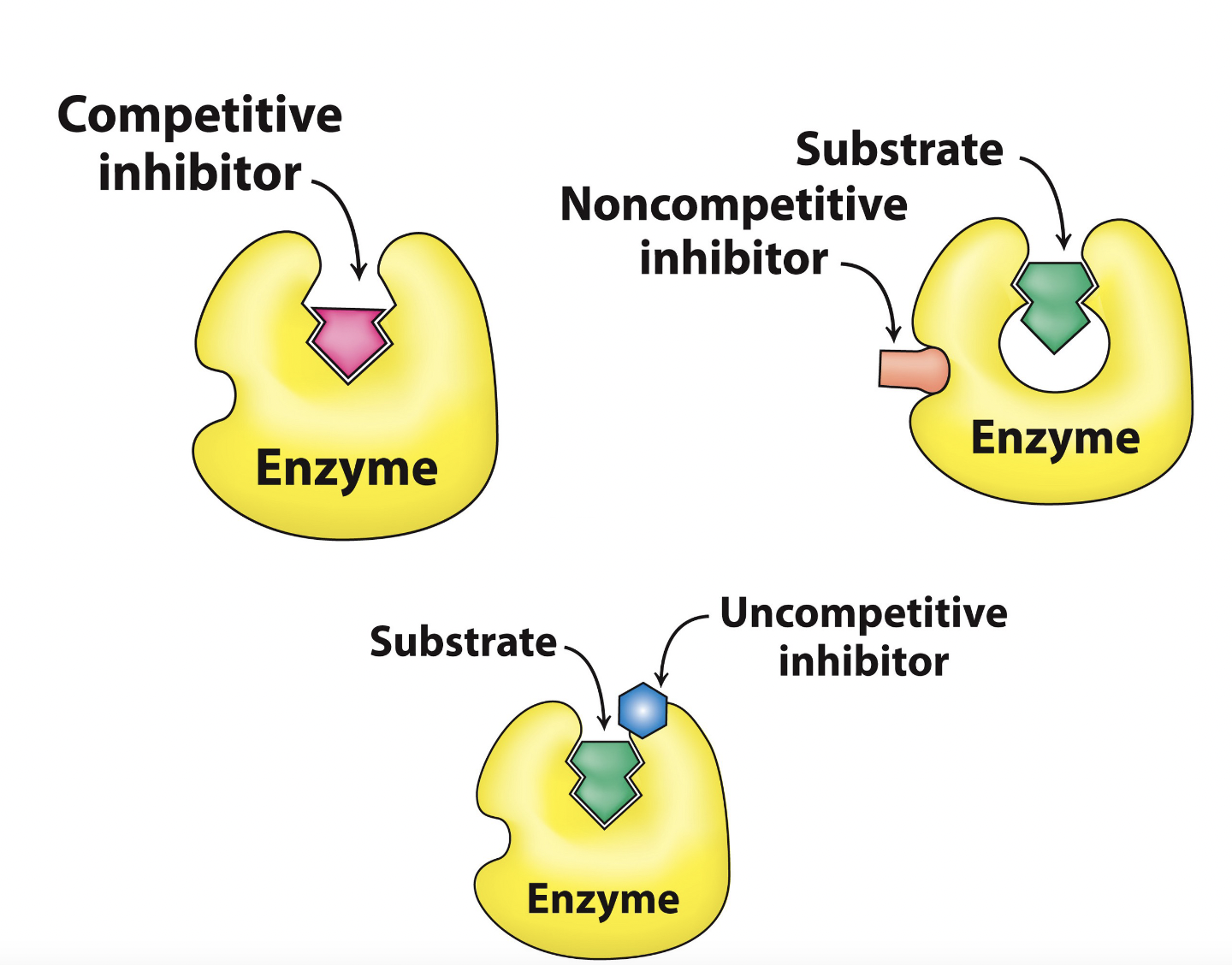
55
New cards
competitive inhibitors
reduces the concentration of free enzyme available \n for substrate binding
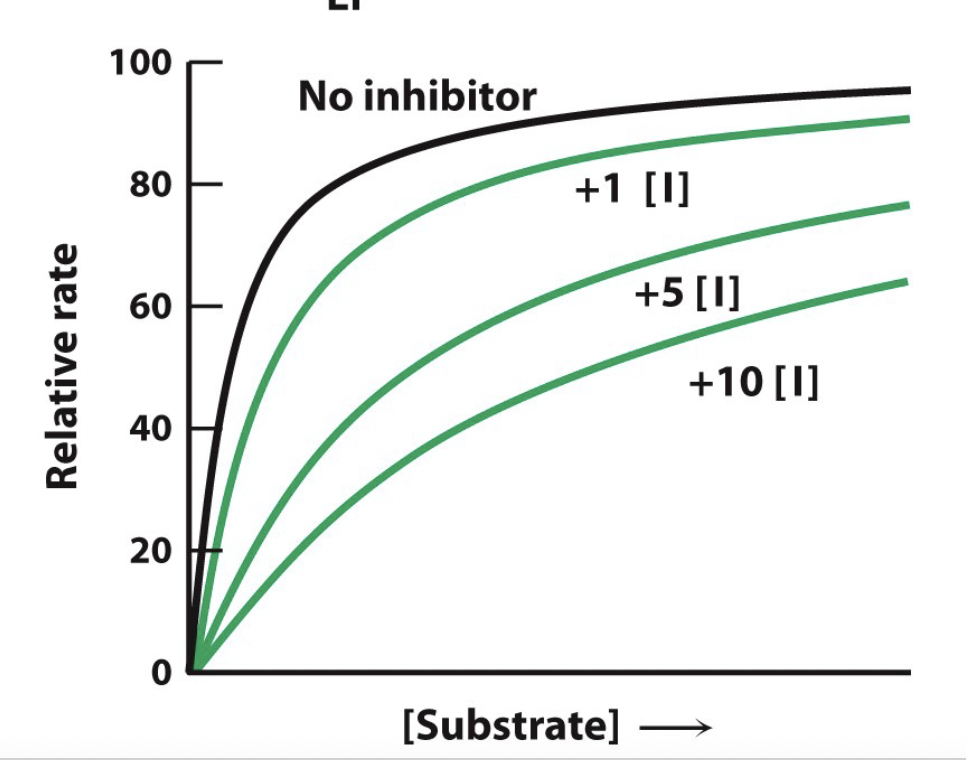
56
New cards
allosteric regulation
molecules that can bind to enzyme at active site, increase or decrease enzyme activity
57
New cards
free energy equation
ΔG = ΔH - TΔS and ΔG^o = -RTln(Keq)
58
New cards
driving force for protein folding and membrane formation
hydrophobic effect
59
New cards
hydrophobic effect
The tendency of water molecules to minimize their \n contact with hydrophobic molecules
60
New cards
buffers
mixtures of a weak acid and its conjugate base that \n resist change in pH when either strong acid or strong base is added
61
New cards
does a strong acid have a high or low pKa?
low
62
New cards
buffer pH range
\+/- 1 of the Pka
63
New cards
Zwitterion
a molecule that possesses both a positive and negative charge (an amino acid at neutral pH is an example)
64
New cards
Isoelectric point (PI)
the pH where an amino acid has a net charge of zero (avg of pk1 + pk2 …)
65
New cards
peptide bond
the bond between amino acids (between C and N), resonance stabilized
66
New cards
peptide
linear polymer of amino acids
67
New cards
dipeptides
two linked amino acids
68
New cards
Oligopeptides
4–20 amino acids
69
New cards
Polypeptides
20 or more amino acids linked together
70
New cards
Almost all peptide bonds are in the trans conformation, what is the exception?
proline linkages
71
New cards
what stabilizes the secondary protein structure
H-bonds
72
New cards
what amino acids may disrupt alpha helices?
Valine, threonine, and isoleucine tends to \n destabilize because of steric clashes
Serine, aspartate and asparagine tend to \n disrupt the helix because of their side chains \n has hydrogen bonding potential
Glycine destabilizes a-helix because absence of \n side chain results in greater freedom of rotation \n
Proline produces a kink in an a-helix because \n cyclic structure occupies space that neighboring \n amino acid would otherwise occupy
Serine, aspartate and asparagine tend to \n disrupt the helix because of their side chains \n has hydrogen bonding potential
Glycine destabilizes a-helix because absence of \n side chain results in greater freedom of rotation \n
Proline produces a kink in an a-helix because \n cyclic structure occupies space that neighboring \n amino acid would otherwise occupy
73
New cards
motifs
combinations of secondary features
74
New cards
domain
independently folded unit within a protein
75
New cards
globular proteins
water soluble proteins that fold into compact structures
76
New cards
fibrous proteins
have repeating secondary structures (like keratin or collagen, coiled coils)
77
New cards
how are tertiary structures stabilized?
hydrophobic effect is the largest contributor, then non-covalent charge-charge interactions (salt bridges) and Van der Waals, then disulfide bonds and metal ions, some steric repulsions as well
78
New cards
how do proteins fold?
through the progressive stabilization of intermediates, entropy and free energy decrease as it folds
79
New cards
Proteins can be denatured reversibly, what does this prove?
proof that tertiary structure of a protein is coming from its sequence
80
New cards
protein folding
Hydrophobic Collapse Model (entropy-driven): Protein collapses rapidly around hydrophobic side-chains with the release of bound, water molecules
Nucleation Model (hydrogen bonding): Neighboring residues in sequence form some element of the native secondary structure (e.g., a-helix) that acts as a nucleus for cooperative folding \n
Van der Waals, Charge-Charge \n
Many small proteins fold spontaneously. Others need assistance.
Nucleation Model (hydrogen bonding): Neighboring residues in sequence form some element of the native secondary structure (e.g., a-helix) that acts as a nucleus for cooperative folding \n
Van der Waals, Charge-Charge \n
Many small proteins fold spontaneously. Others need assistance.
81
New cards
Intrinsically Disordered Proteins (IDP)
inherently unstructured proteins, tend to participate in regulation and signaling mechanisms
82
New cards
myoglobin role
Facilitates oxygen diffusion in \n the muscle cells and functions as a oxygen storage \n protein in aquatic mammals
83
New cards
where does oxygen bind in myoglobin?
prosthetic heme group
84
New cards
role of proximal histidine
makes Fe stay in the Fe2+ state
85
New cards
hemoglobin
transports oxygen throughout the body, does not have a heme group because it needs to be able to both take in and drop off oxygen
86
New cards
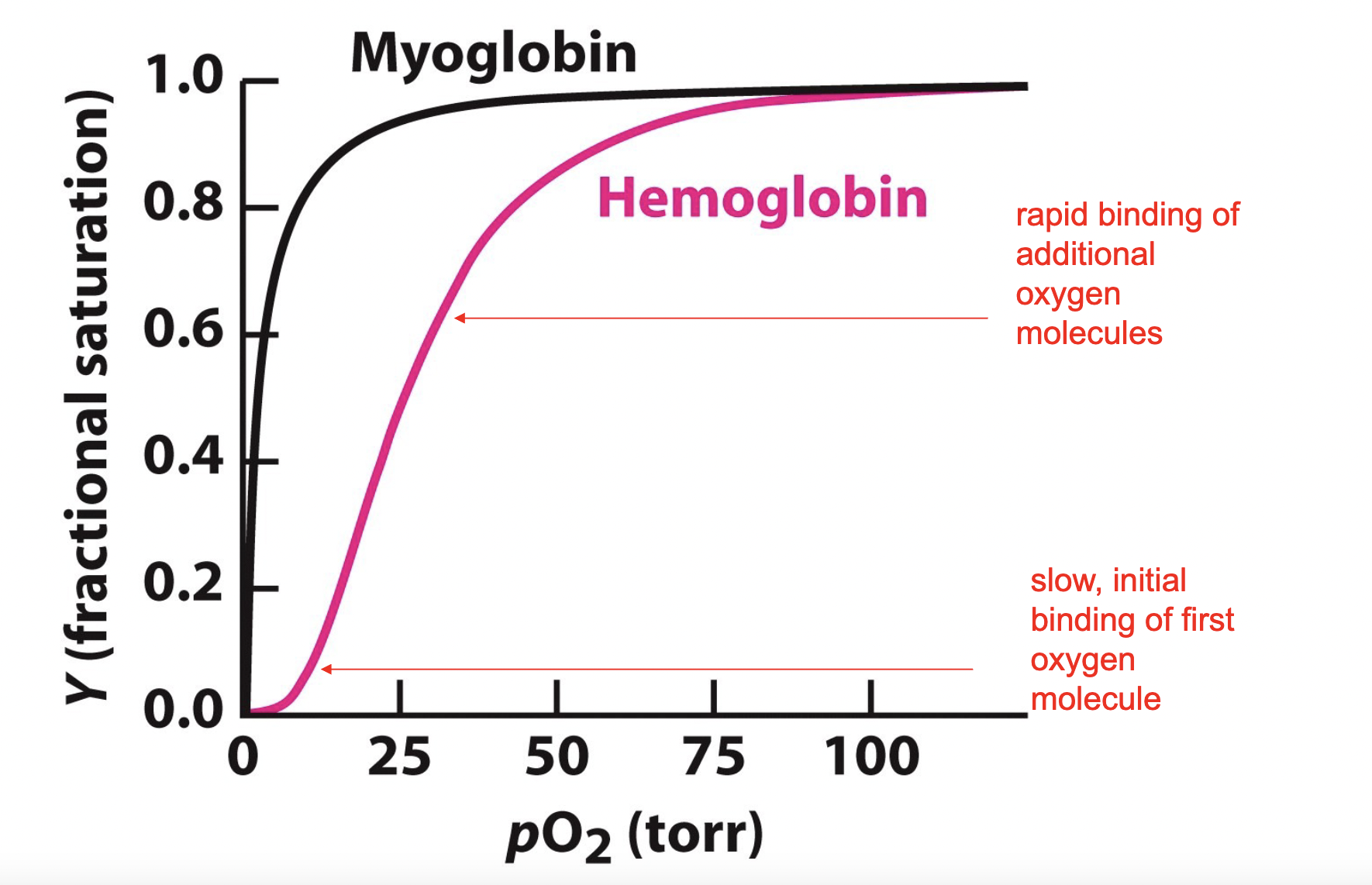
what is this graph
oxygen binding curve for hemoglobin and oxygen, more oxygen needed to saturate hemoglobin, hemoglobin exhibits cooperative binding
87
New cards
hemoglobin states
T-state: not bound to O2
R-state: bound to O2
R-state: bound to O2
88
New cards
how does cooperativity work?
Initial binding of first O2 shifts Fe+2, proximal His and its associated a-helix toward the plane of the porphyrin ring
89
New cards
How does 2,3- BPG effect hemoglobin binding?
it binds to allosteric sites and stabilized deoxygenated hemoglobin, so it increases oxygen unloading capacities at the tissue level
90
New cards
Bohr effect
lowered pH decreases hemoglobin affinity to bind to O2, thus offloading ability of hemoglobin increases (seen at the tissue level)
91
New cards
fetal hemoglobin
has higher oxygen binding affinity that that of the mother
92
New cards
proteins must be what in order to be purified?
proteins must be released from the cell in order to be purified
93
New cards
ways to purify a protein
by solubility, size/mass, charge, binding affinity,
94
New cards
cryo-electron microscopy
direct images of proteins (sum of photos from different directions), good for large proteins
95
New cards
what are nucleotides composed of?
nitrogenous base, sugar, and a phosphate
96
New cards
role of both DNA and RNA
store and decode genetic information
97
New cards
properties of nitrogenous bases
Aromatic, planer, and heterocyclic molecules
98
New cards
what are the purines?
Adenine (A) and Guanine (G)
99
New cards
what are the pyrimidines?
Cytosine (C), Thymine (T), and Uracil (U)
100
New cards
phosphodiester linkages
ester bonds that form between sugar and phosphate to form the backbone of nucleic acids.