Redox
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
Oxidation Number rules
Metal Hydrides (e.g LiH, CaH2): Hydrogen = -1
Peroxides (e.g H2O2): Oxygen is -1
2
New cards
Oxidation (electrons)
Loss of electrons
Oxidation number becomes more positive
3
New cards
Reduction (electrons)
Gain of electrons
Oxidation number becomes more negative
4
New cards
Oxidising agents
Accept electrons from the species that is being oxidised
Therefore gains electrons and is reduced
5
New cards
Reducing Agents
Donate electrons to species being reduced
Therefore loses electrons and is oxidised
6
New cards
Disproportionation reactions
Species is both oxidised and reduced
Both increase and decrease in oxidation number for that species
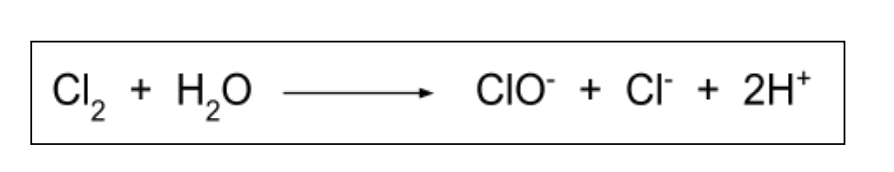
7
New cards
Redox reactions w/ Metals
Metal + Acid → Salt + Hydrogen