Pharmacogenomics
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Pharmacogenetics and pharmacogenomics
Pharmacogenetics
The study of the relationship between individual gene variants and drug effects
Pharmacogenomics
The study of the relationship between variants in a large collection of genes (up to the whole genome) and drug effects
PGX defined as “The molecular study of genetic factors that determine drug efficacy and toxicity”
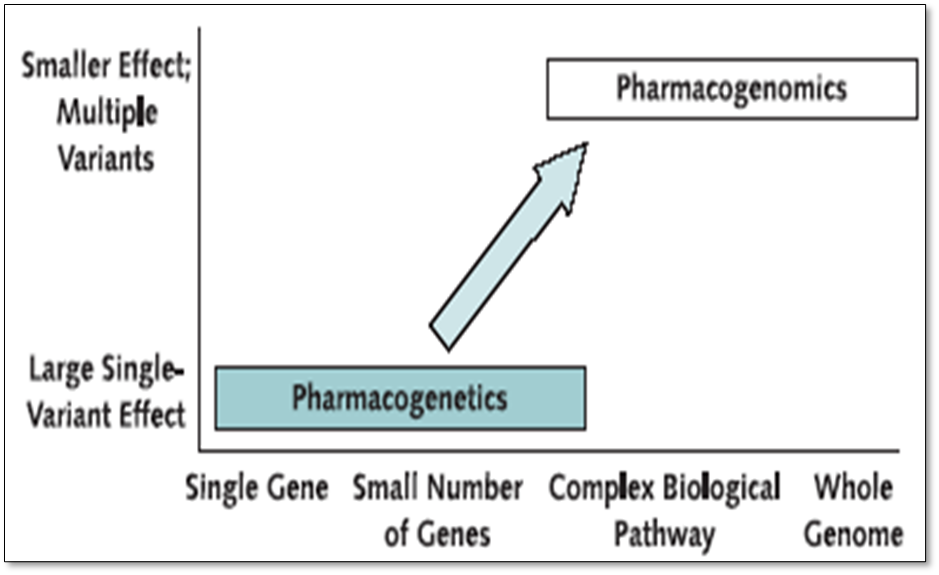
Research has been going on for more than decade and uses classical genetic techniques such as twin studies and patterns of inheritance at the single gene level. This commonly involves classical genetics approach. - looking for an unusual drug response or metabolism phenotype and then conducting a family study to understand the pattern of inheritance and identify the gene responsible.
Pharmacogenetics is the study of single genes or a small number of genes and these genes will have a large effect on the phenotype.
Pharmacogenomics relates to a wide genome approach - understanding how genetic variations affect the way people respond to drugs, typically involves large scale studies. Explores the way these variations can be used to predict whether a patient will have a good response, bad response or nor response to a drug and consideration of all the inherited variations in genes that relate to a drug response.
For majority of phenotypes there are going to be a number of genes involved in the same there are a number of genes involved in complex disorders.
One size does not “Fit all”
Many factors influence an individuals’ response to a drug (both beneficial and harmful)

Age - function of liver and kidney can become impaired as you get older, leading to drugs accumulating
Gender - Role of hormones, differences between pre and post menopausal woman. Pre-menopausal women tend to metabolise drugs faster than men
Weight - dose dependant on weight
Disease state - chronic disorders of the liver and kidney will effect drug metabolism and excretion
Ethnicity
Environmental factors
Polypharmacy and drug drug interactions - coadministration of drugs can lead to induction or inhibition of cytochrome p450 which can affect concentration of drug
diet - presence of food can impact the absorption of drugs, grapefruit juice leads to higher circulation of drugs metabolised by this enzyme.
lifestyle - alcohol increases sedative effects
Genetic variation
First recognised in 70s where it was found that some people had chromosome for cytochrome p450 enzymes isoform zip 2d6 showed a functionally important variation in its DNA - some people had an inactive form meaning drs had to optimise regimen by process of trial and error
Currently optimisation of dosage for an individual patient is mainly a process of trial and error.
This can expose a patient to adverse drug reactions or reduced efficacy in some cases
What are the potential benefits OF PGX?
•Personalised / stratified / precision medicine
•Focused treatments by identification of people likely to respond
identifying a person’s genotype and using that information to predict outcomes.
It's about using the medicines that we have to greater precision the use of the best drug available for the purpose.
•Minimising ADRs by predicting people at risk
•Promising drugs in the pharmaceutical pipeline that show ADRs in certain groups of the population may be “saved”
•Identification of those with an unmet clinical need for future drug development
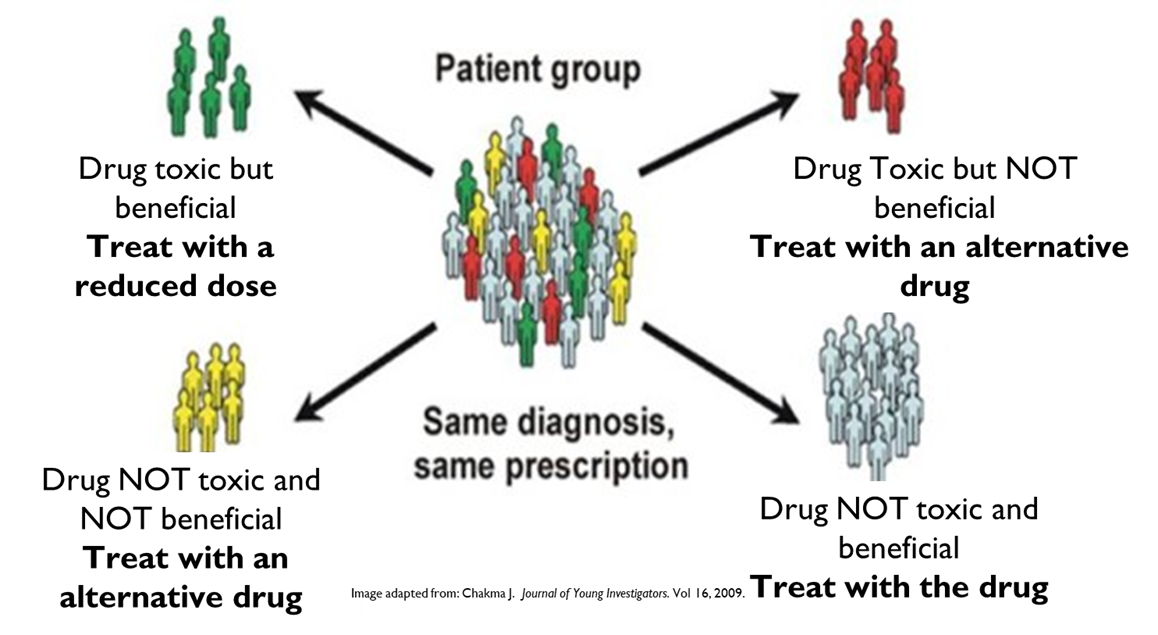
The application of pharmacogenomics should improve the outcomes for patients because it can improve the effectiveness of pharmacotherapy by allowing the development of personalised drug treatments regimens for individuals that use the most appropriate therapy as an optimised dose. This can have the benefit of improving adherence. If you knew a drug would work for you you would be more inclined to use and accept the ADR associated with it.
Pharmacogenomics can also have an effect on the drug development process. Currently one in 20 drugs make it through phase 1 of the clinical trial process due to a lack of effect in a significant population or a serious side effects in a few people. The stratification of patient cohorts based on their genotypes could lead to the use of drugs in a subgroup of people over population.
Adverse drug reactions
•~3.5 % of hospital admission are a consequence of ADRs to prescription drugs.
•Leads to ~200,000 fatalities per year in Europe
•Most common major ADRs:
Cardiovascular
Liver failure
Severe dermatological hypersensitivity
•Many other minor problems:
Minor rash
Gastrointestinal discomfort
Dry mouth
Drowsiness
Headache
Many adverse drug reactions are a consequence of variations in genes associated with pharmacokinetics, higher cost to also now manage the side effects.
The potential benefit of adjusting dose to PK genotype
Uniform drug exposure would reduce dose dependant ADRs and prevent therapeutic failure when efficacy cannot be immediately assessed
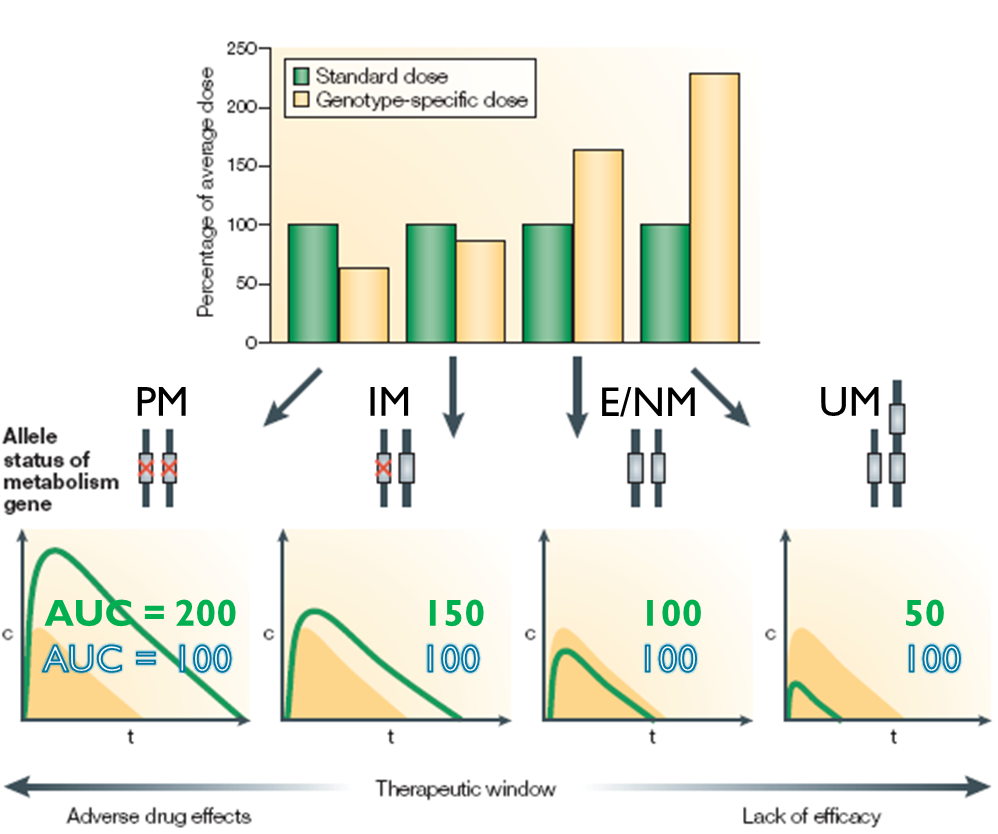
Figure illustrates the potential benefit of adjusting the dose to genotype, difference in clearance and the AUC - variation in drug concentration in blood plasma. This can be overcome by drug adjustments.
Here the poor metaboliser (PM) has two non functional alleles, IM has one non functional and 1 intermediate, NM has 2 functional and UM has an extra copy of the gene by gene amplification and has a faster than normal metabolised status.
Looking at graph green is standard dose and yellow is desired AUC
If we look at the difference between PM and UM she’d get a quarter of the dose of the UM. Uniform drug exposure would reduce dose dependant ADR and prevent therapeutic failure.
Predictions of estimated dose adjustments required for PK genotype
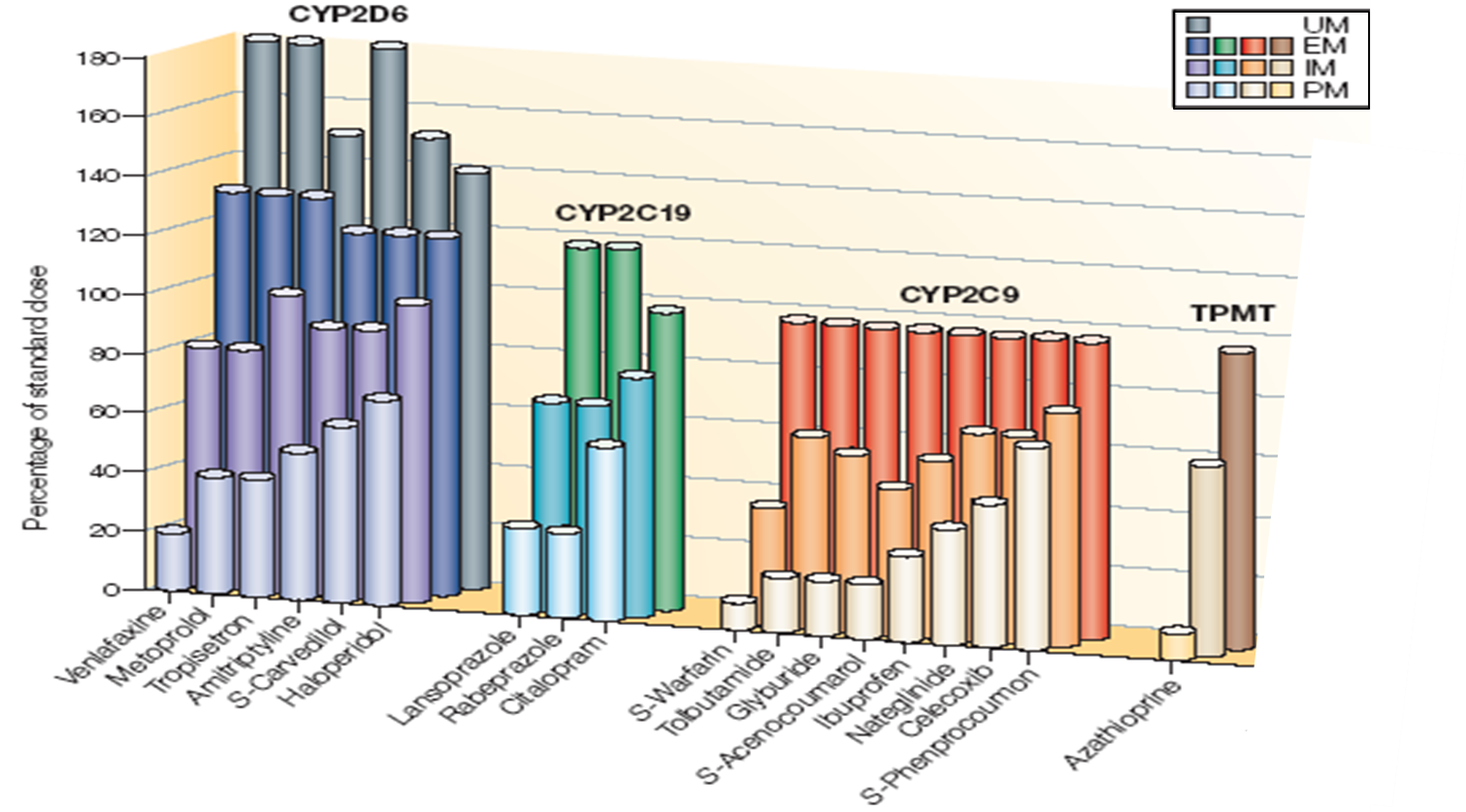
This slide shows predictions of estimated dose adjustments that are required for some important genes involved in drug metabolism. TheY have variations that lead to different phenotypes. The percentage of the standard dose for commonly used drugs that metabolised by the different genes based on genotype are shown.
The four enzyme shown are CYP2D6 which has four phenotypes from poor to ultra and has a number of anti-depressants as substrates. CYPD2C19 19 has PPI and SSRI as substrates.
CYP2C9 has NSAID’s and warfarin as substrate. A ll of these have three metabolites - PM, IM, and E/NM.
Then you have TPMT which has 3 metabolised phenotypes and 5 purine substrates.
You can see that there's a large dose adjustment required to achieve the same levels of drug exposure in individuals with the different genotypes, the same dose of drug could yield poor or no effect, an extensive or ultra metabolises or toxic response in poor metabolises.
Cytochrome P450s
Human genome contains 57 genes for these enzymes but less than 12 of them are responsible for the oxidative metabolism of almost all drugs.
•Important drug metabolising enzyme involved in phase 1 oxidative metabolism of xenobioitcs
There are many allele variations within different populations and several variations are associated with drug effectiveness.
•Almost all hepatic CYPs are POLYMORPHIC
•2 to 4 metaboliser phenotypes are common (Poor, Intermediate, Extensive / Normal and Ultra)
•Most important enzyme subtypes where alleles are associated with altered drug response:
•CYP2C9 (warfarin)
•CYP2C19 (PPIs, clopidogrel)
CYP2D6 (codeine, tamoxifen, antipsychotics, antidepressants
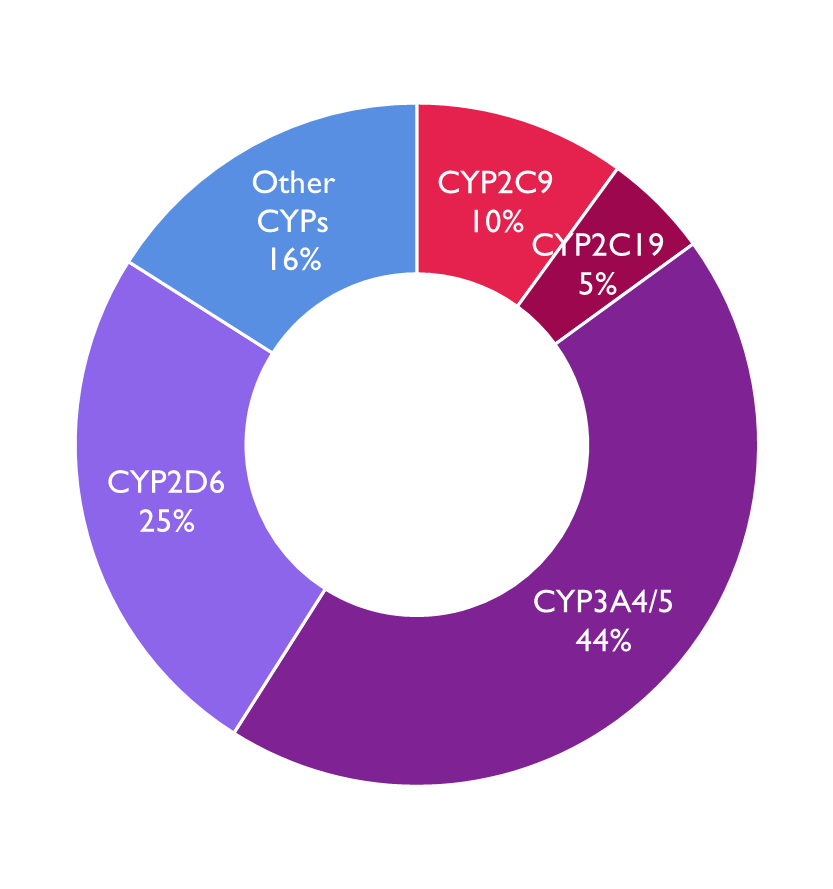
Image shows % of drugs that are metabolised by the different CP450 enzymes
CYP2D6
•~25% of drugs metabolised by CYP2D6
•PMs may suffer from ADRs at the usual doses of drugs with narrow therapeutic
•2.5-6 fold increased risk of risperidone ADRs (e.g. tardive dyskinesia etc)
•5 fold increase in risk of ADRs with metoprolol (vertigo, disturbed sleep etc)
•Do not respond to prodrugs that require activation by this enzyme e.g. codeine
•PM phenotype can also be a consequence of CYP2D6 inhibition by a drug interactions (phenocopy) eg SSRI inhibition
UMs
May suffer from toxicity from prodrugs that require metabolic activation
Do not reach the therapeutic dose
Prevalence of poor and intermediate metaboliser phenotype in different populations
Phenotype | Population | Allele | % |
Poor | Caucasian | *4 | 5-10% |
African | *4 | 6-7% | |
Asian | *4 | <1% | |
Intermediate | Asian | *10 | 50% |
Caucasian | *10 | 10-15% | |
African | *17 | 9%-34% | |
Caucasian | *17 | <1% |
Its important to know the metabolising status of individual for some drugs so we can genotype them
In this table you can see that some alleles for PM and IM phenotypes have different prevalence in different ethnic groups as shown in this table. Therefore it is important to know which allele to look for within each population group. A pharmacogenomic test may be different in Asia compared to one in Europe.
Codeine & UM CYP2D6 status
Genotype differences can have serious consequences if we look at the prevalence of the UM status CYP2D6. We can see that it varies from one to 29 percent within different population groups.
Codeine is a prodrug metabolised by this enzyme into the active metabolite morphine.
•Contraindicated in
•women during breastfeeding - UM may have higher levels of the active metabolites in breast milk and on very rare occasions may result in symptoms of opioid toxicity in the infant, which may be fatal.
•In patients known to be ultra-rapid metabolisers -increased risk of developing adverse effects of opioid toxicity even at commonly prescribed doses.
Population | Prevalence UM phenotype (%) |
African/ Ethiopian | 29 |
African American | 3.4 to 6.5 |
Asian | 1.2 to 2 |
Caucasian | 3.6 to 6.5 |
Greek | 6.00 |
Hungarian | 1.90 |
Southern European | 10 |

Case report here shows a woman who was given codeine after giving birth to her child. She was BF her baby who after 11 days died of respiratory collapse. The child was found to have 4 times the normal amount of morphine in its circulations, The mother was not a metaboliser leading to high concentration of morphine in her breast milk. Her child was an extensive stabiliser, but was unable to metabolise the drug effectively as neonates have a lower adme ability.
Coumarin anticoagulant therapy
•Warfarin - effective anticoagulant - used as a thromboprophylatic but has a narrow therapeutic index and serious ADRS

•Important to keep clotting rate in correct range for those taking warfarin (INR 2-3).
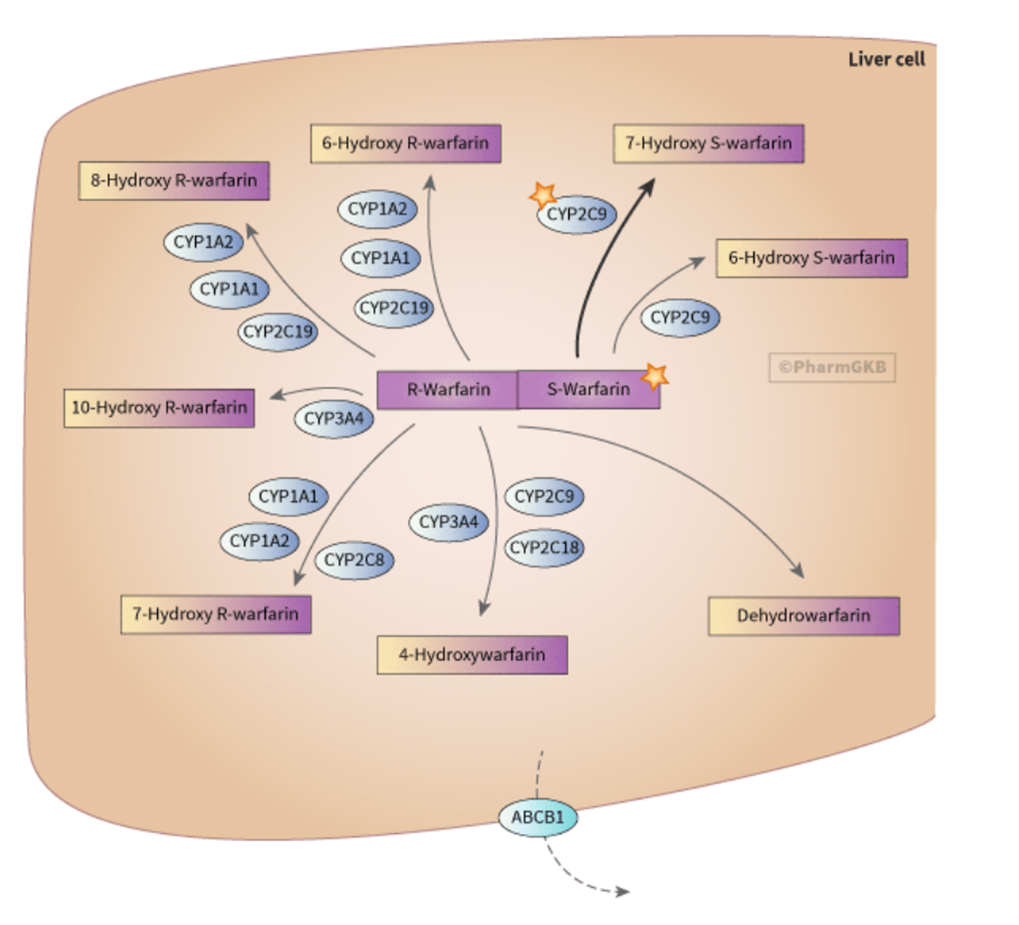
Warfarin Pharmacokinetics
•S-Warfarin is 3-5 times more potent than R-Warfarin
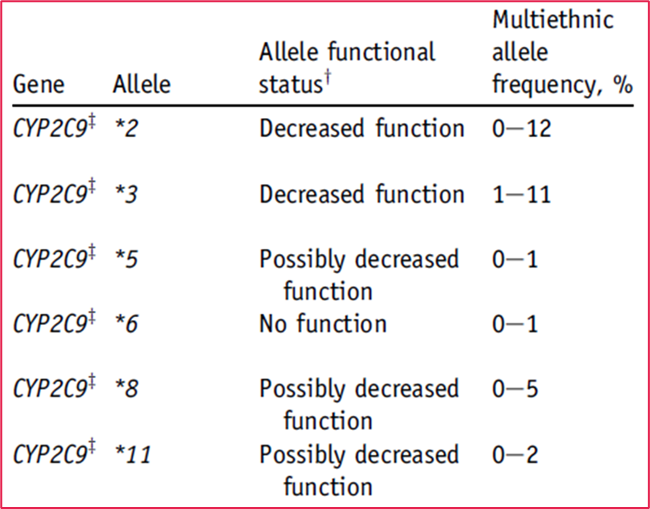
•CYP2C9 is the principle isoform responsible for metabolism of the S isomer and is polymorphic with 3 phenotypes
There are a number of PM alleles with different frequencies in different populations
individuals with low CYP29 activity would be expected to have a lower warfarin requirement as the dose stays around in their system for longer.
Warfarin PHarmacodynamics
•Warfarin achieves its anticoagulant effect by inhibiting the enzyme vitamin K epoxide reductase (VKORC1 gene) - reduces Vitamin K to biologically active Vitamin K
Reduced Vitamin K acts as a cofactor for a carboxylase that produces functionally active proteins - Factor 9 and 10
•VKORC1*2 allele (-1639 G>A) has a SNP in the promotor region within the transcription factor binding site.
•“A” allele associated with reduced expression of the enzyme and reduced dose phenotype
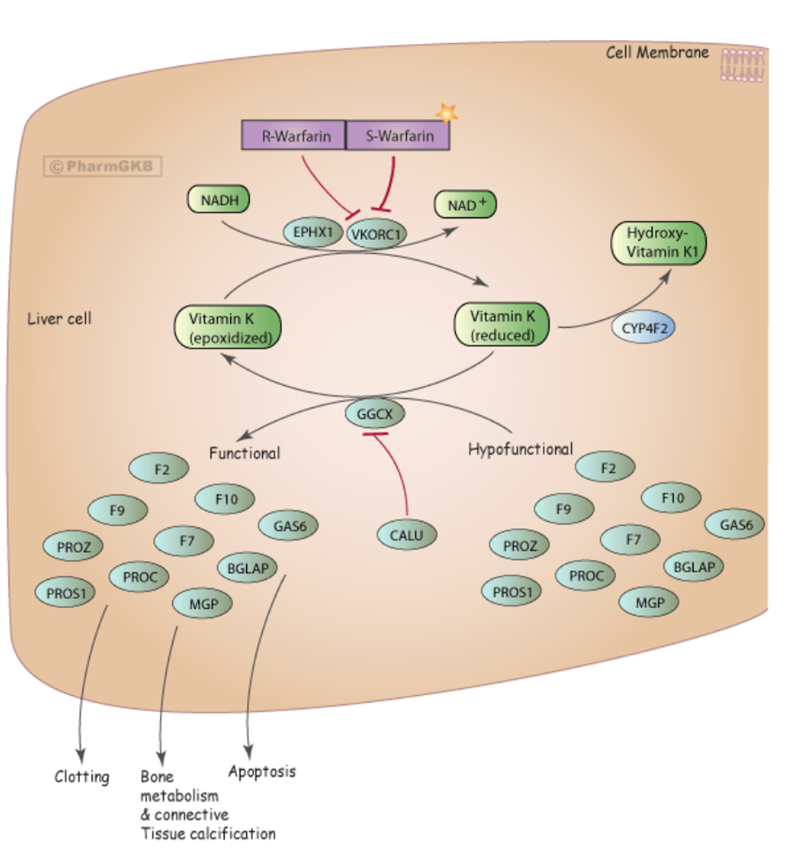
Warfarin PGX
•The combination of these two genotypes overall is important
•Suggested dosage for warfarin (mg/d) as a function of CYP2C9 and VKORC1 genotypes ranges from 0.5 to 7 mg/d
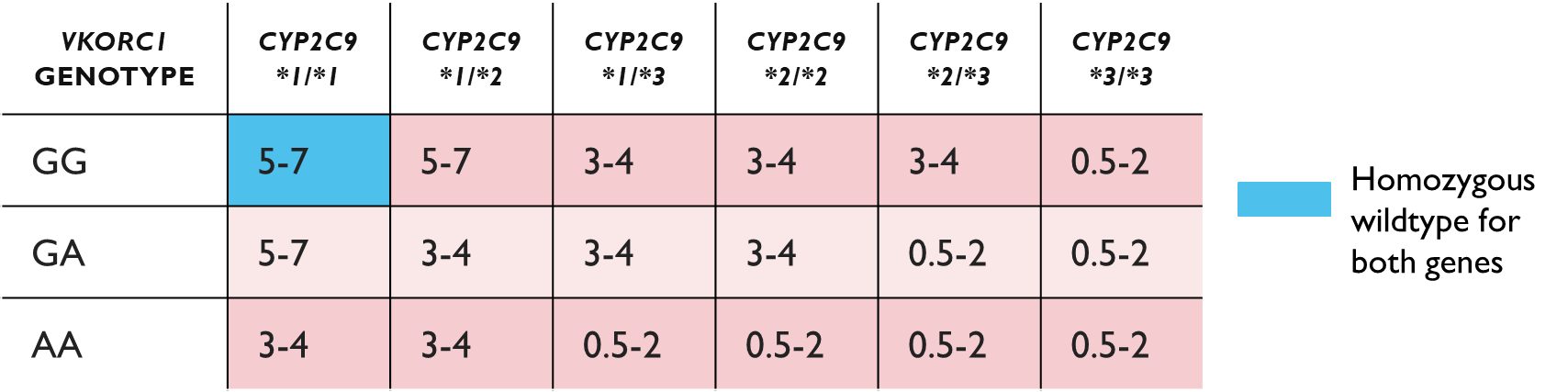
•The inclusion of genotyping in the warfarin doing algorithm can
•Improve prediction of the loading dose of warfarin
•Improve prediction of the maintenance dose when used with INR data
•See Fitzgerald et al., 2019 for further information on the implementation of a point of care genotype guided dosing
Drug induced hypersensitivity
•Can just affect the skin or in combination with systemic effects (e.g. liver toxicity)
•Can cause blistering of the skin
•Steven-Johnson syndrome (SJS) 10% of skin affected
•Overlap syndrome 10-30% of skin affected
•Toxic epidermal necrolysis (TEN) > 30% of skin and 2 mucous membranes affected
•Inappropriate immune reaction where the drug is acting as the antigen leading to activation of the adaptive immune response
•Associated with the MHC locus on chromosome 6 - HLAB5701 (see the screencast on biomarker identification for information on Abacavir)
•Population incidence: 1 - 10%
DRUG INDUCED HYPERSENSITIVITY - Carbamazepine
•Anticonvulsant used in the treatment of epilepsy and bipolar disorder
•Causes rashes in 10% of Patients, hypersensitivity syndrome and can rarely lead to SJS or TEN
•SJS is associated with HLA-B*1502 allele
•Incidence: Han Chinese (10-15%) Thai (2-4%), African, Caucasian (<1%)
•Testing recommended for those of Asian ancestry
Thiopurine S-methyltransferase (TPMT)
•Thiopurine S-methyltransferase (TPMT) is a phase II enzyme required for the inactivation of thiopurines which are used clinically as antimetabolites to supress the immune system and also in cytotoxic chemotherapy.
•Thiopurines e.g. 6-mercaptopurine are converted into active metabolites (thioguanine nucleotides) which are incorporated into DNA and inhibit DNA replication
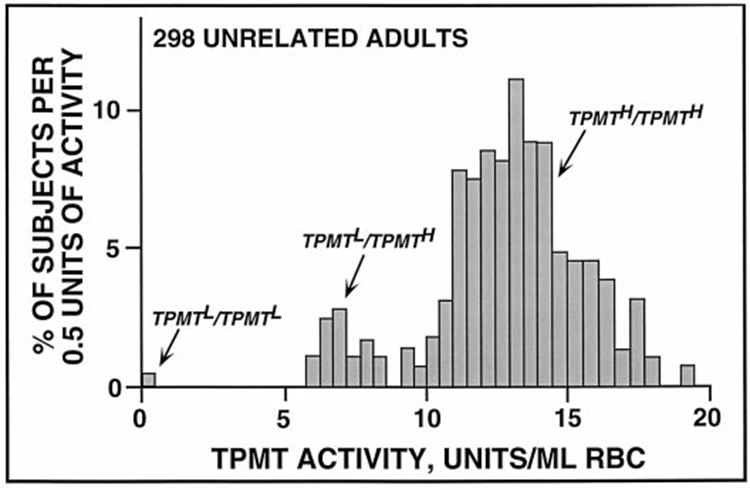
•Exhibits co-dominant allelles with a trimodal phenotype in Caucasian and African American populations:-
1.~ 89% have normal activity of enzyme (H/H) - Homozygous wildtype, can range from person to person from between 10 to 20 units of activity per ml of RBC
2.~ 11% have intermediate activity (heterozygous H/L) - activity in the range of 6 to 8 units
3.0.39% (1 in 300) have very low TPMT activity(L/L)
•In Asians less than 5% of the population have at least one defective allele
•Other enzymes are also important for thiopurine toxicity e.g. A purine-specific nucleotide diphosphatase (NUDT15)
Low activity SNPs
•Three missense variations rs1800462 (G>C), rs1142345 (A>G) and rs1800460 (G>A) account for 95% of individuals with reduced TPMT activity. This important because Thiopurine associated myelotoxicity is associated with low enzyme activity
The L/L population has a higher risk of life threatening haemopoietic toxicity with potential septic complications with potential septic complications when treated with conventional doses of thiopurines - therefore not a good candidate for thiopurine therapy
those with heterozygous genes will have Intermediate activity so the respond to therapy but are still at risk of myelotoxicity. Reducing the dosage minimises this risk.
Those with homozygous wild type allele require 4 fold higher doses of 6 mercaptopurine to have a therapeutic effect. if they are not given that then there is more risk of relapse of their condition.
•The variant alleles result in significant decreases in levels of TPMT protein or its activity
•Actionable PGX - Determining TPMT status is recommended before treatment
•Guidelines on genotyping and dosing have been published (PHARMGKB) suggesting alternative medications for L/L and 50% dose for H/L heterozygous patients.
uridine diphosphoglucyronyl transferase (UGT1A1)
•Uridine diphosphoglucyronyl transferase (UGT1A1) is a enzyme involved in phase II metabolism
•Responsible for the inactivation by glucuronidation of the active metabolite SN-38 of topoisomerase inhibitor, used as a cytotoxic chemotherapy agent. Irinotecan
•> 60 alleles
•The UGT1A1*28 allele (TA)7 repeat in TATA box of its promoter has a low activity phenotype (70% lower gene expression)
RIGHT - basically UGT1A1 is an enzyme that helps get rid of certain drugs including irinotecan which is a prodrug used to kill cancer cells. However it can also affect normal cells so its very important that this drug is cleared out properly. Some people have the *28 alleles which means in the promoter region they have 7 repeats of TA instead of 6 making the gene less active and therefore the enzyme doesn’t work as well. Therefore they cannot clear the metabolite (sn-38) as well so more stays in the body - can lead to very low WBC count - neutropenia
UGT1A1 genotype and colorectal cancer chemotherapy
•Effect of genotype on ADR determined for 3 chemotherapy combinations
•IFL - Fluorouracil & Irinotecan
•FOLFOX – Flurouracil & Oxaliplatin
•IROX – Irinotecan & Oxaliplatin (double the irinotecan dose)
•
••Actionable PGX - A reduced initial dose is recommended for *28 (TA)7 homozygotes
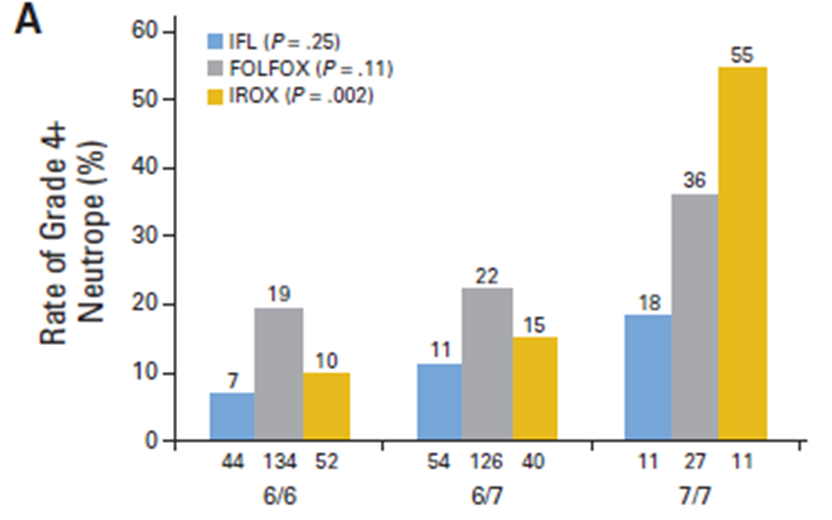
The UGT1A128 variant slows down irinotecan breakdown.
Patients with two copies (7/7) are at much higher risk of severe toxicity, especially at higher irinotecan doses (like in IROX).
Pharmacogenomic testing helps identify them so doctors can reduce the initial dose and make treatment safer.
Gefitinib
•A small molecule inhibitor targeted against the tyrosine kinase domain of the epidermal growth factor receptor (EGFR / HER1)
•Binds to the ATP binding site of the tyrosine kinase domain and down regulates receptor autophosphorylation and phosphorylation of downstream molecules
•Has anti-tumour activity in a subgroup of patients
Gefitinib sensitising genotypes
•Greater efficacy is seen in those with mutations in the kinase domain of the receptor either a small deletion or a single base change where leucine
is changed to arginine.
•
•The SNP (L858R) or inframe deletions in exon 19 are predictive of a response to Gefitinib
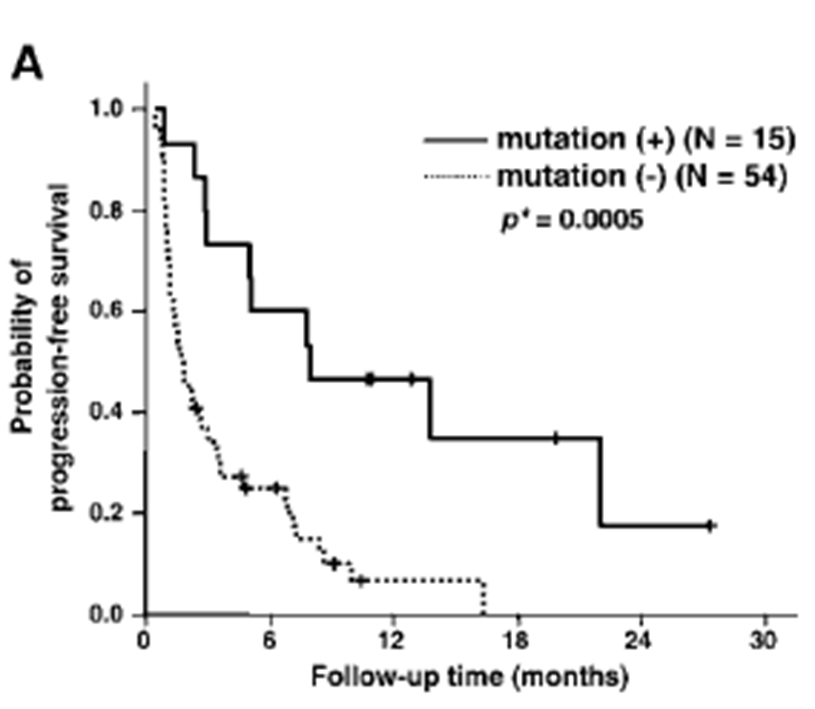
Survival stratified by L858R genotype
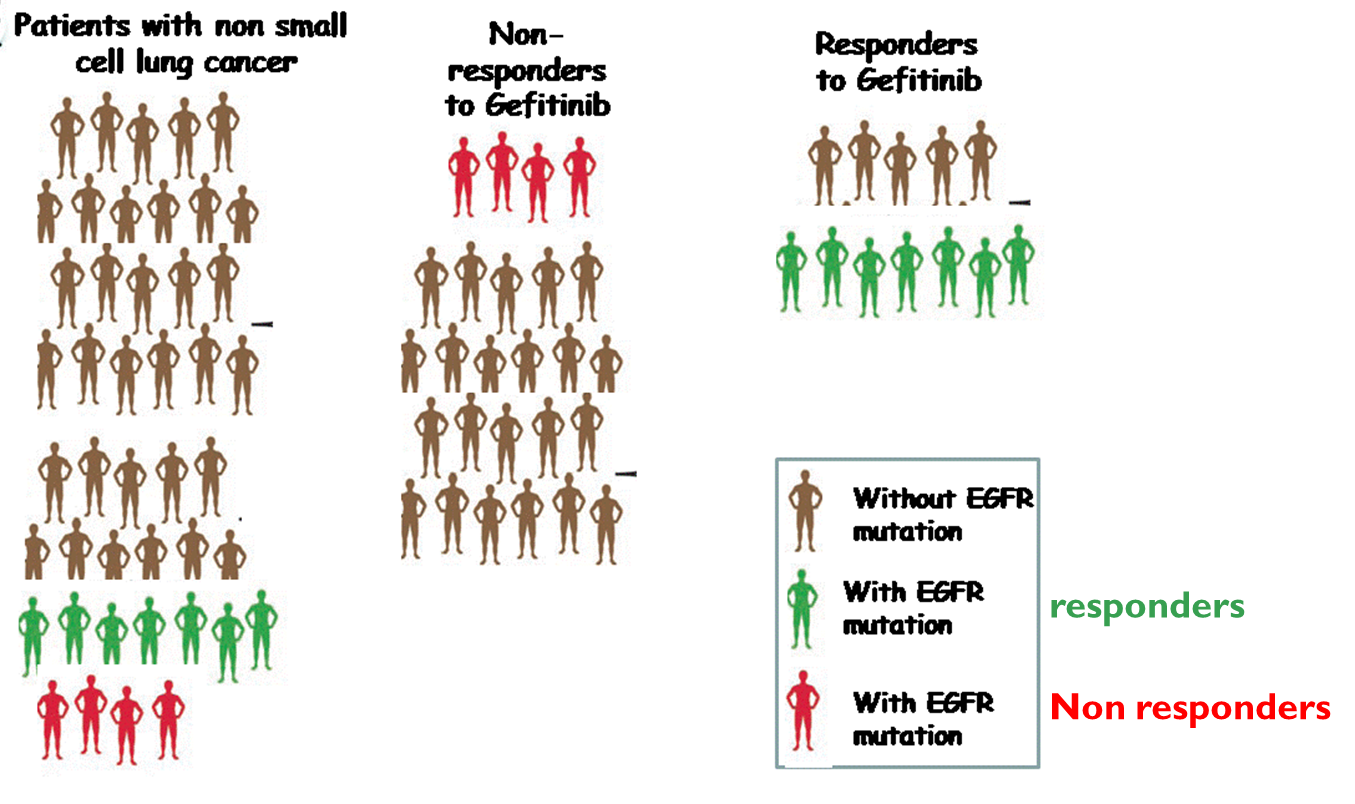
EGFR mutation is not wholly predictive for response to Gefitinib
EGFR signalling pathway
Gefitinib resistant genotypes
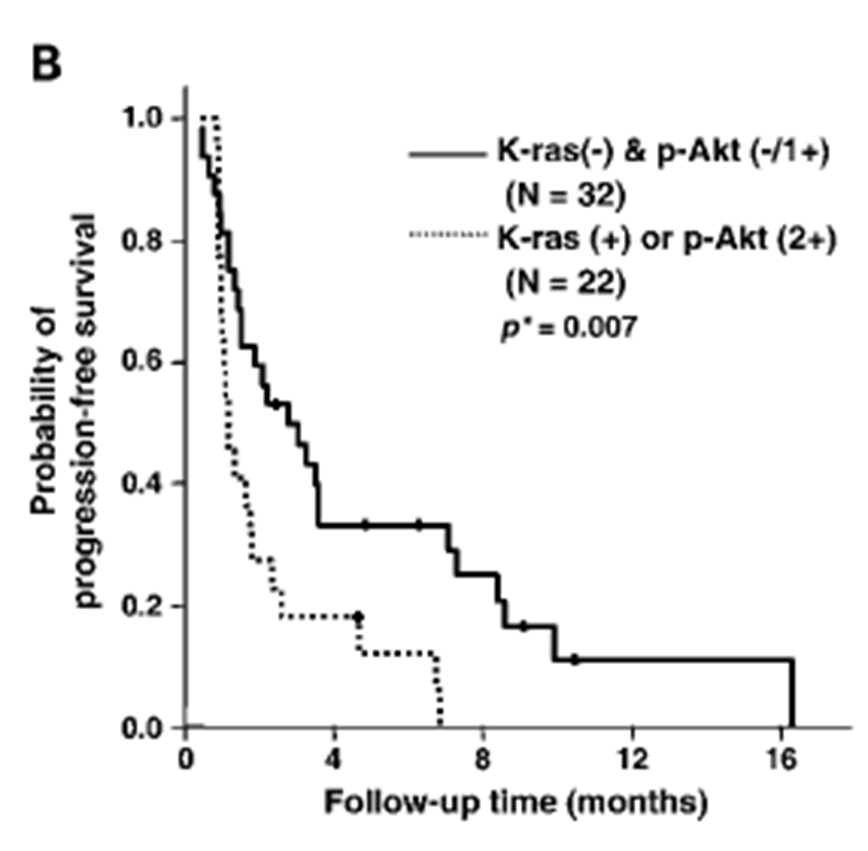
Presence of K-ras mutation or p-Akt overexpression is associated with disease progression
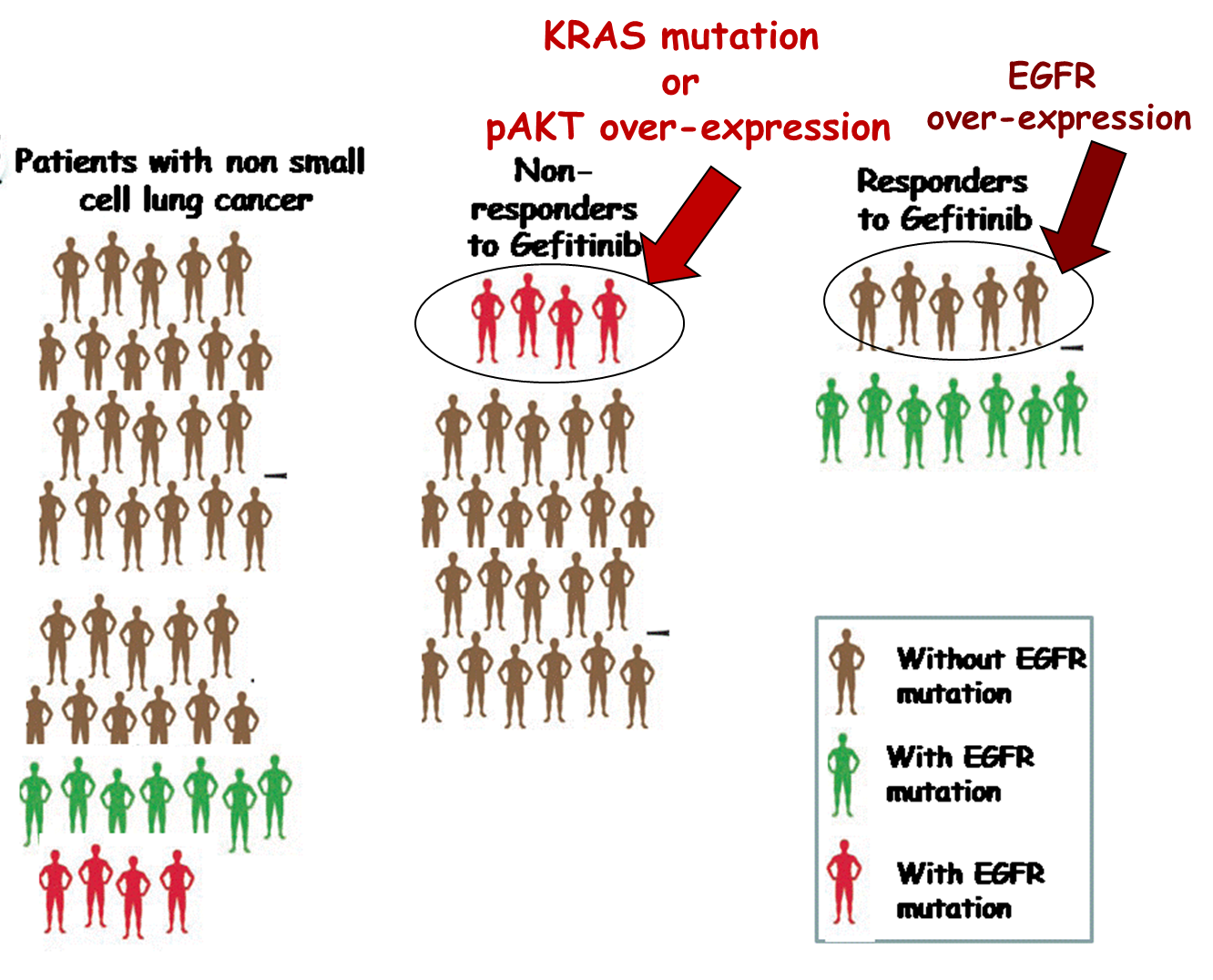
Gefitinib resistant genotypes
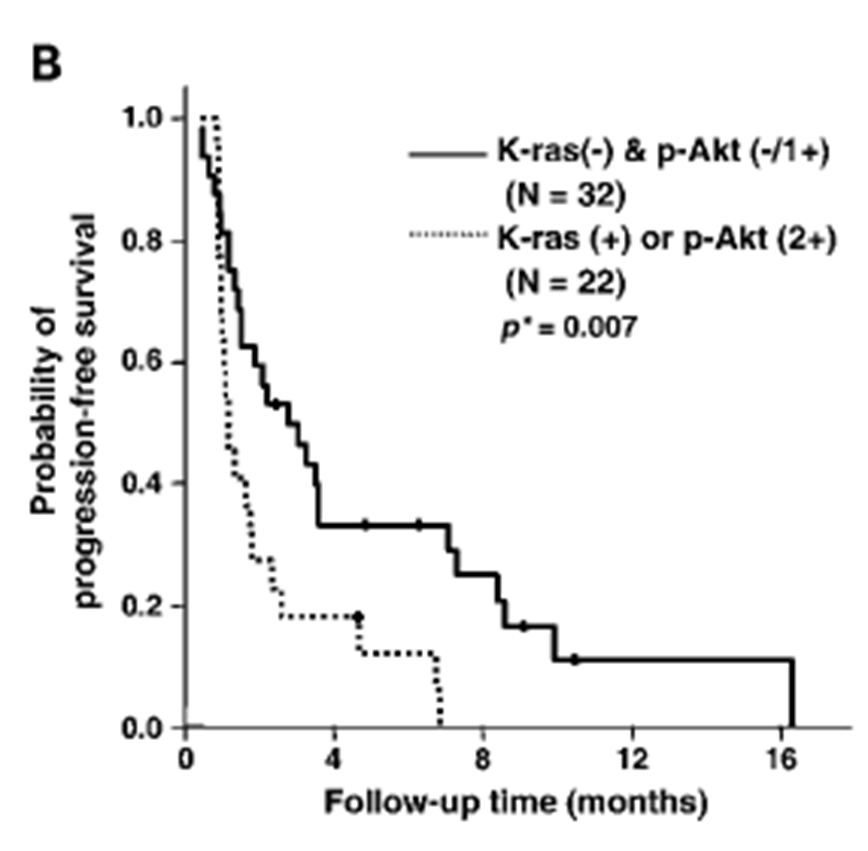
Presence of K-ras mutation or p-Akt overexpression is associated with disease progression
Graph basically shows progression of cancer - Those with the mutation has a better prognosis and those with wild type receptor has reoccurrence within 16 months., whereas 20 percent of those with mutation were still progression free after 28 months
EGFR mutation is not wholly predictive for response to Gefitinib
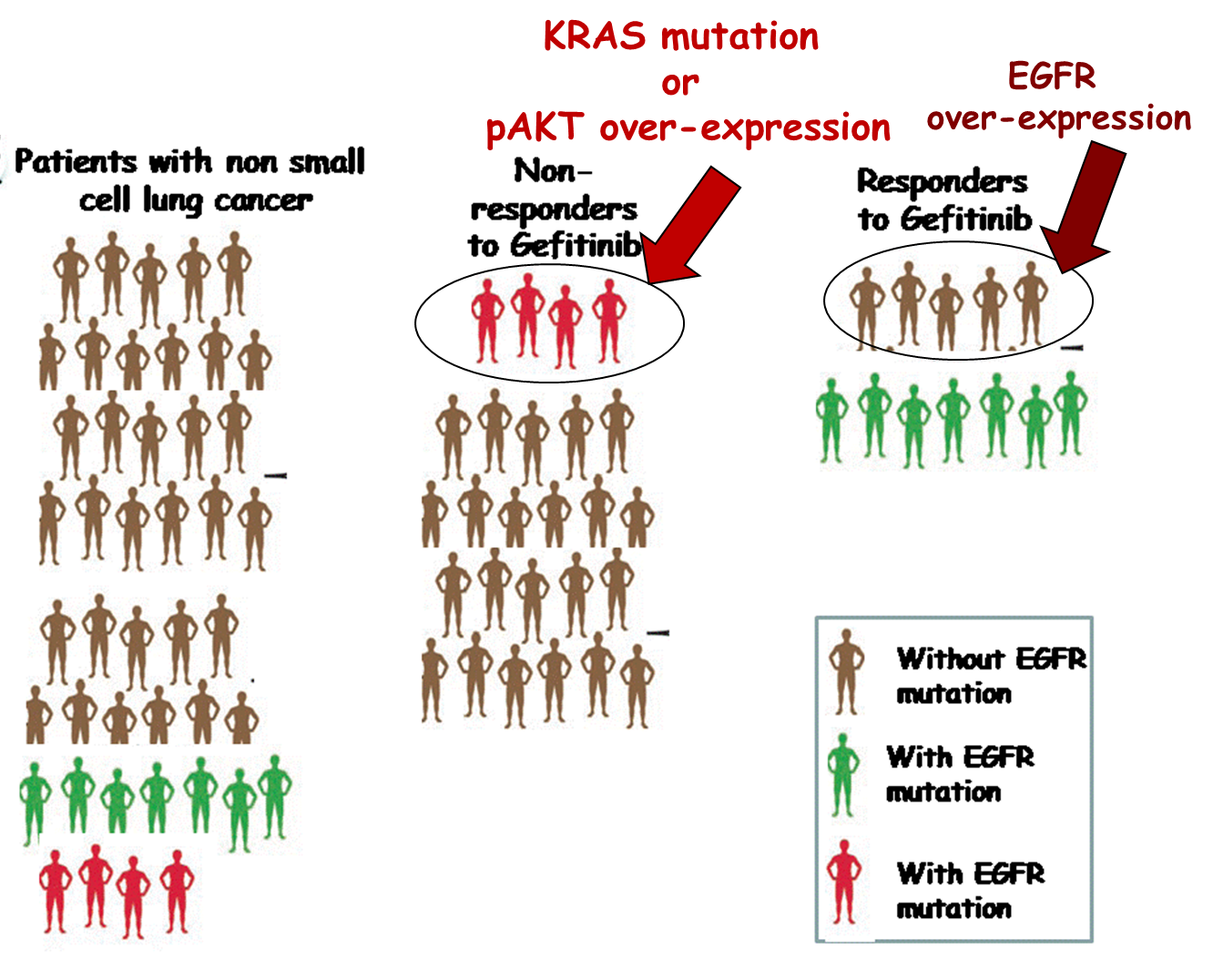
Graph shows that not everyone who responded to gefitinib actually had the mutations
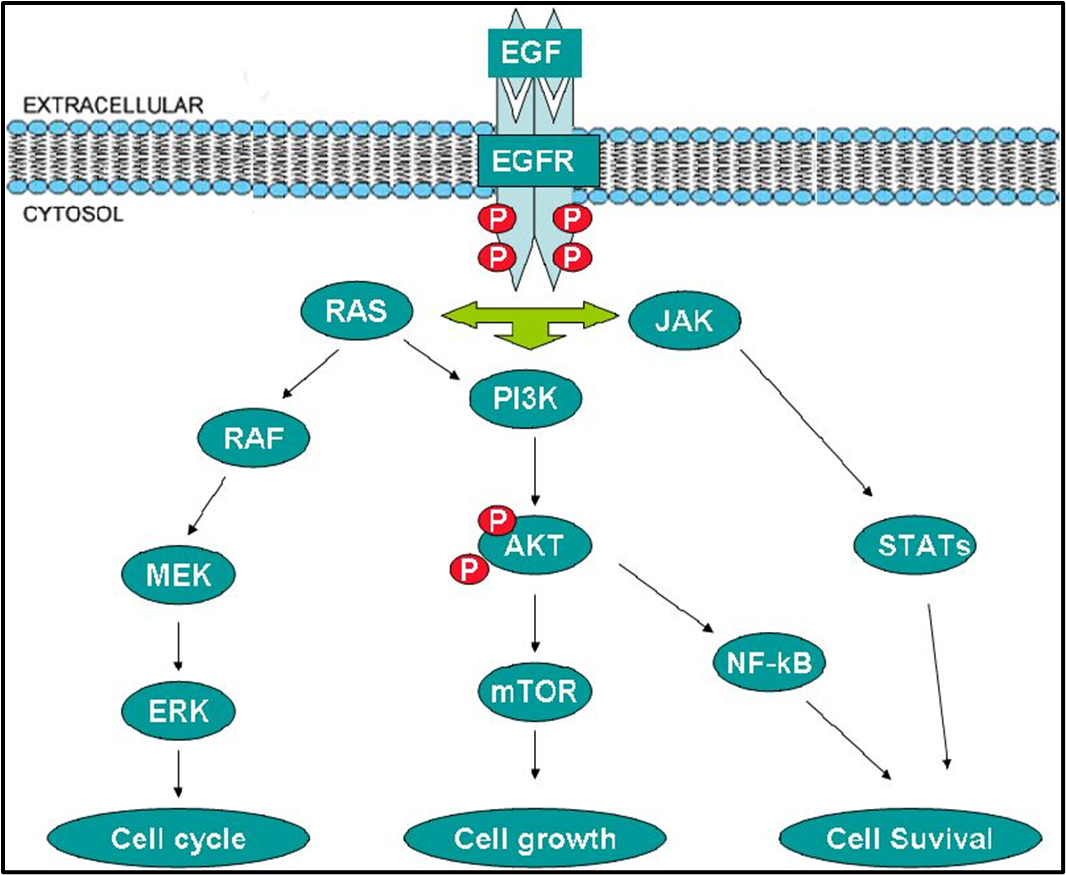
EGFR SIGNALLING PATHWAY
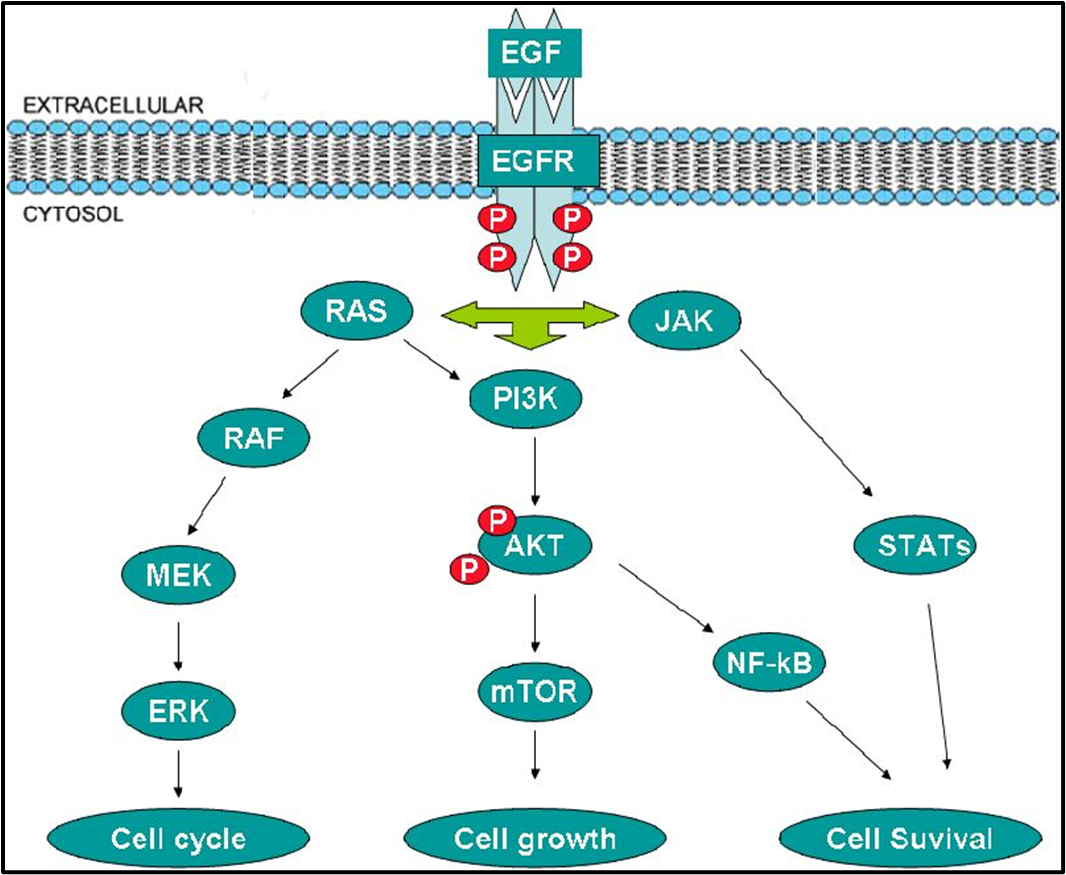
Gefitinib binds to epidermal growth factor receptor tyrosine kinase domain leading to down regulation of the receptor autophosphorylation
There are a number of signal transduction pathways associated with EGFR
basically it activates RAS and a bunch of other stuff which controls cell division but overactivation of this can lead to uncontrolled cell growth - cancer
gefitinib blocks the receptor
Use of Gefitinib in the UK
•Pharmacodynamics - Indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with activating mutations of EGFR-TK (Exon 19 deletions; L858R)
•Pharmacokinetics - CYP3A4 predominantly and also CYP2D6
•CYP3A4 inhibitors + CYP2D6 PM status = increased risk of adverse effects
•
•Acquired resistance to Gefitinib – T790M mutation in the kinase domain (exon 20) led to the development of 3rd generation TKI - Osimertinib
TraSTuzumab
•Trastuzumab is an example of the benefit of PGX
•Identification of those with an unmet clinical need for future drug development
•Promising drugs in the pharmaceutical pipeline that show ADRs in certain groups of the population may be “saved”
