PATHO – Hematologic Disorders Flashcards 🧬 – IRAT 7 (Part 2)
1/162
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
163 Terms
🧬 Macrocytic Anemias – Flashcards
What are the 2 types of Macrocytic Anemias?
Folate Deficiency
Vitamin B12 Deficiency
What defines a macrocytic anemia?
🔹 MCV > 100 um³
→ This means red blood cells are larger than normal.
What are the most common causes of macrocytic anemia?
🌿 Folate deficiency
💊 Vitamin B12 (cobalamin) deficiency
These cause megaloblastic anemia — where RBC precursors are large due to impaired DNA synthesis.
Why do folate or vitamin B12 deficiencies cause megaloblastic anemia?
Because both are needed for DNA synthesis
when DNA synthesis slows, the nucleus develops slowly but cytoplasm grows normally → cells become large and immature (megaloblasts).
💡 Think: “Big cells = DNA stuck in slow motion.”
What is another name for macrocytic anemia caused by folate or B12 deficiency?
📚 Megaloblastic anemia — due to impaired DNA synthesis in BM.
What are other causes of macrocytic anemia (not megaloblastic)?
🍷 Alcoholism
🫀 Liver disease
💊 Drugs (e.g., 5-fluorouracil, hydroxyurea, zidovudine)
💡 These cause macrocytosis without megaloblasts — via membrane or metabolism effects.
What is the role of folate (Tetrahydrofolate, THF) and vitamin B12 (cobalamin) in red blood cell (RBC) formation, and how is methionine formed from THF?
💡 Folate and B12 = DNA-building helpers.
Both are required to convert homocysteine → methionine, which is needed for DNA synthesis.
THF gives a methyl group → B12 activates it → methyl group added to homocysteine → forms methionine → methionine used for DNA synthesis in RBCs.
Lack of folate or B12 = impaired DNA synthesis → large, immature RBCs.
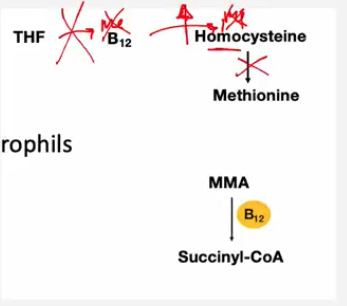
What happens if there is a THF (folate) or B12 deficiency?
🔺 Homocysteine builds up (can’t convert to methionine).
🔺 DNA synthesis slows down, leading to large, immature RBCs (megaloblasts).
🔺 Causes macrocytic, megaloblastic anemia.
What happens to the erythroblasts (RBC precursors) in folate or B12 deficiency?
They undergo impaired cell division, leading to:
Fewer RBCs produced
Larger than normal precursors
Macrocytic, hypochromic RBCs in circulation
Besides RBCs, which other blood cell line is affected in macrocytic anemia, and how?
Granulocytes (especially neutrophils) are affected.
Impaired DNA synthesis leads to delayed nuclear maturation, causing hypersegmented neutrophils (≥ 5 lobes).
💡 Hypersegmented neutrophils = classic clue for folate or B12 deficiency.
How can you differentiate folate vs. B12 deficiency?
Feature | Folate Deficiency | B12 Deficiency |
|---|---|---|
Neurologic symptoms | ❌ Absent | ✅ Present (paresthesia, ataxia) |
Cause | Diet, alcoholism, drugs | Pernicious anemia, malabsorption |
Lab | ↑ Homocysteine | ↑ Homocysteine + ↑ Methylmalonic acid |
💡 B12 = “Brain + Bone Marrow,” affects both.
Describe Macrocytic Anemia to a child in one sentence:
“Macrocytic anemias (MCV > 100) are usually caused by folate or B12 deficiency → impaired DNA synthesis → large, immature megaloblasts. Other causes include liver disease, alcoholism, and certain drugs.
What is the difference between erythroblast and granulocyte changes seen in macrocytic anemia?
Cell Type | Defect | Result |
|---|---|---|
Erythroblasts | Impaired division | Large RBC precursors → macrocytic anemia |
Granulocytes | Impaired division | Hypersegmented neutrophils (>5 lobes) |
What are the main dietary sources of folate?
Green leafy vegetables 🥬
Some fruits 🍊🍓
💡 Folate = “foliage” — think green leaves!
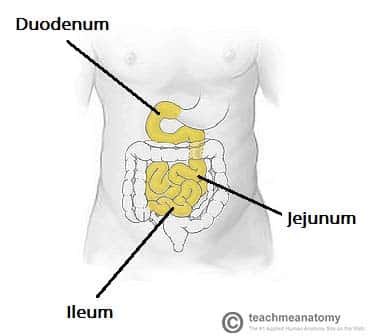
Where in the gastrointestinal tract is folate absorbed?
Jejunum (middle portion of the small intestine)
How quickly does folate deficiency develop, and why?
Develops over months
Because body folate stores are limited and turnover is fast (unlike B12, which takes years to deplete)
Which conditions or situations cause an increased demand for folate?
Pregnancy 🤰 (fetal growth)
Children (rapid cell division)
Cancer (increased cell turnover)
Hemolytic anemia (accelerated RBC production)
💡 Any condition with fast-growing or replacing cells increases folate demand.
What factors lead to poor intake of folate?
Alcoholism (interferes with folate absorption and diet quality)
Elderly individuals (poor diet, decreased absorption)
What drugs can cause folate deficiency, and how do they do it?
Folate antagonists, such as methotrexate (MTX)
They inhibit dihydrofolate reductase, an enzyme needed to convert folate → active form (THF)
💊 MTX = blocks folate activation → ↓ DNA synthesis.
What is the overall result of folate deficiency on red blood cells?
impaired DNA synthesis → megaloblastic (macrocytic) anemia
Large, immature RBCs in bone marrow and peripheral blood
🧠 Summary Flashcard:
Folate comes from green veggies & fruits, absorbed in the jejunum, and is easily depleted.
Deficiency develops over months due to increased demand, poor intake, or folate-blocking drugs (e.g., methotrexate) → leading to megaloblastic anemia.
What type of anemia results from folate deficiency, and what is its hallmark feature on a blood smear?
Megaloblastic macrocytic anemia
Hallmark: Large, immature RBCs (megaloblasts) + hypersegmented neutrophils (>5 lobes)
💡 DNA synthesis is impaired → cell division slows → large cells form.
What are the main symptoms of folate deficiency?
Fatigue, weakness, pallor (due to anemia)
Glossitis (smooth, red tongue) *know
No neurologic symptoms (this differentiates it from B12 deficiency)
How does folate deficiency affect serum lab values?
Test | Folate Deficiency | Explanation |
|---|---|---|
Serum folate | ⬇ Decreased | Folate depleted |
RBC folate | ⬇ Decreased | Long-term folate status |
Homocysteine | ⬆ Increased | Folate needed to convert it to methionine |
Methylmalonic acid (MMA) | 🟢 Normal | Folate doesn’t affect MMA (helps differentiate from B12 deficiency) |
Why are neurologic symptoms absent in folate deficiency?
Because folate is not directly involved in myelin synthesis, whereas vitamin B12 is.
🧠 → Folate deficiency causes anemia only, not neuropathy.
🧠 Summary Flashcard
Folate deficiency → megaloblastic anemia (big, immature RBCs) + hypersegmented neutrophils.
Labs: ↓ folate, ↑ homocysteine, normal MMA.
No neurologic symptoms (unlike B12 deficiency).
💉 Vitamin B12 (Cobalamin) Deficiency
Where does dietary vitamin B12 come from?
Found in animal-derived foods 🥩🐟🥚
Bound to animal proteins in food
💡 Vegetarians and vegans are at higher risk of deficiency.
What happens to B12 first when it enters the mouth?
Salivary enzymes (especially amylase) release B12 from food proteins.
Then B12 binds to R-binder (haptocorrin) — a glycoprotein made in saliva that protects it in the stomach’s acidic environment.
🧠 Think: R-binder = “Rescue binder” from stomach acid.
What happens to the B12–R-binder complex in the duodenum?
Pancreatic proteases (from the pancreas) break R-binder apart.
This releases B12, allowing it to bind to intrinsic factor (IF).
💡 Without pancreatic enzymes, B12 stays stuck to R-binder — can’t be absorbed!
What is intrinsic factor (IF), and where is it produced?
A glycoprotein produced by parietal cells of the stomach.
Essential for B12 absorption — it binds B12 after R-binder is cleaved.
💡 IF = “Important Factor” for B12 absorption.
Where in the GI tract is the B12–intrinsic factor complex absorbed?
Absorbed in the terminal ileum (last part of the small intestine).
What organs or enzymes are required for normal B12 absorption to occur?
Stomach (parietal cells) → make intrinsic factor
Salivary glands → secrete R-binder
Pancreas → releases proteases to free B12
Terminal ileum → absorbs B12–IF complex
💡 If any step fails → B12 deficiency develops.
Describe B12 Deficiency in simple terms.
🥩 B12 (from animal foods) → bound to R-binder in stomach → released by pancreatic enzymes in duodenum → binds intrinsic factor → absorbed in terminal ileum.
🔁 Requires: Saliva, Stomach, Pancreas, and Ileum working together.
How does B12 deficiency differ from folate deficiency in terms of frequency and timing?
A:
B12 deficiency is less common than folate deficiency.
It takes years to develop because the liver stores large amounts of B12.
What type of diet can lead to B12 deficiency, and why?
Strict vegan or vegetarian diets, since B12 is found only in animal-derived foods (meat, fish, eggs, dairy).
Deficiency appears after several years of dietary restriction.
What is pernicious anemia, and how does it cause B12 deficiency?
Autoimmune destruction of parietal cells in the stomach → loss of intrinsic factor (IF).
Without IF, B12 cannot be absorbed in the terminal ileum.
How does gastrectomy or bariatric surgery cause B12 deficiency?
Removes or damages parietal cells, leading to intrinsic factor deficiency.
Also decreases acid production, which is needed to release B12 from food.
How does pancreatic insufficiency lead to B12 deficiency?
A: Lack of pancreatic enzymes prevents proper B12 processing and absorption.
Pancreatic proteases normally cleave B12 from R-binder in the duodenum.
Without these enzymes, B12 cannot bind to intrinsic factor → malabsorption.
💡 Think: R-binder = “locked”; pancreas = “key.”
What intestinal condition can cause B12 deficiency by damaging the absorption site?
Conditions like Crohn’s disease damage the ileum, where B12 is absorbed.
What causes B12 Deficiency? (*4)
B12 deficiency is less common, develops slowly, and is caused by:
Pernicious anemia (↓ IF)
Pancreatic insufficiency
Ileal damage (Crohn’s)
Vegan diet (rare dietary cause)
What type of anemia is caused by B12 deficiency?
Megaloblastic macrocytic anemia (large, immature RBCs).
What are two main clinical signs or symptoms of B12 deficiency?
Glossitis (smooth, inflamed tongue) 👅
Subacute degeneration of the spinal cord 🧠 (due to demyelination)
What neurologic symptoms can occur in B12 deficiency?
What causes the neurologic symptoms in B12 deficiency?
Subacute combined degeneration of the spinal cord →
Numbness
Tingling (paresthesias)
Difficulty walking (ataxia)
Loss of position/vibration sense
Build-up of methylmalonic acid (MMA) in the myelin of the spinal cord, leading to demyelination → weakness, numbness, poor coordination.
How are serum B12 levels affected in B12 deficiency
How are homocysteine levels affected in B12 deficiency?
How are methylmalonic acid (MMA) levels affected in B12 deficiency?
Serum B12 levels: Decreased (↓)
Homocysteine levels: Increased (↑)
Methylmalonic acid (MMA) levels: Increased (↑)
How do serum lab values appear in B12 deficiency?
Test | Finding | Explanation |
|---|---|---|
Serum B12 | ⬇ Decreased | Deficient stores |
Serum Folate | Normal | Not affected |
Homocysteine | ⬆ Increased | B12 required to convert it to methionine |
Methylmalonic acid (MMA) | ⬆ Increased | B12 required to convert MMA → succinyl-CoA |
💡 ↑ MMA helps distinguish B12 from folate deficiency
How do blood cells appear on a smear?
How do bone marrow findings appear?
Macrocytic RBCs
Hypersegmented neutrophils (>5 lobes)
Megaloblastic changes in erythroid and granulocyte precursors (large, immature nuclei).
✅ Summary Card:
B12 deficiency → megaloblastic macrocytic anemia + neurologic symptoms.
Symptoms: Glossitis, subacute spinal cord degeneration
Blood smear: Macrocytic RBCs + hypersegmented neutrophils
Labs: ↓ serum B12, ↑ homocysteine, ↑ MMA (MMA buildup damages myelin)
Onset: slow due to large liver stores.
🩸 Normocytic Anemia – Overview Flashcard
What are the main 6 causes of normocytic anemia, and what do they have in common?
Hereditary spherocytosis (membrane defect)
Sickle cell disease (HbS polymerization)
Paroxysmal nocturnal hemoglobinuria (PNH) — complement-mediated intravascular hemolysis
G6PD deficiency (oxidative hemolysis)
Immune hemolytic anemia (antibody-mediated)
Microangiopathic hemolytic anemia (shearing in small vessels)- (MAHA)
All have—> normal-sized RBCs (MCV 80–100 µm³) but decreased RBC count
What defines normocytic anemia?
A1: Anemia with a normal MCV (80–100 µm³) — red blood cells are normal in size but fewer in number.
What are the two main causes of normocytic anemia?
Increased peripheral destruction of RBCs (hemolysis)
intravascular hemolysis (destroy RBCs in vasculature)
extravascular hemolysis (in heart, spleen, mechanical valve)
Decreased production of RBCs (bone marrow underproduction)
What does increased peripheral destruction mean?
RBCs are being destroyed faster than they can be replaced — either:
Intravascularly (inside vessels) → e.g., PNH, G6PD deficiency
Extravascularly (in spleen/liver) → e.g., hereditary spherocytosis, sickle cell disease
What does underproduction refer to in normocytic anemia?
The bone marrow isn’t producing enough RBCs — due to causes like chronic disease, renal failure (↓EPO), or bone marrow suppression.
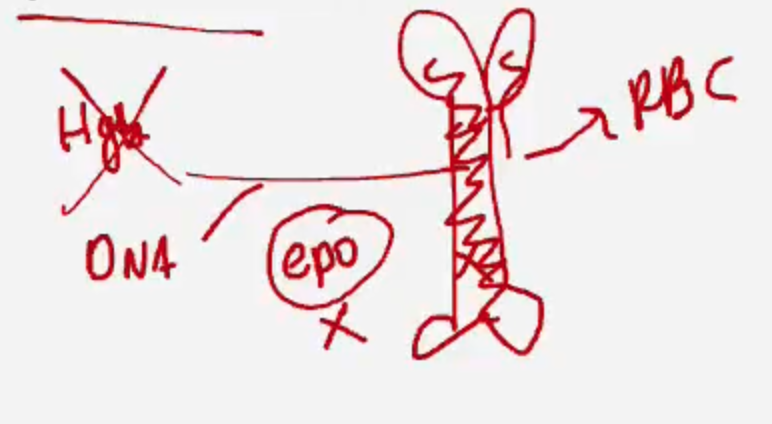
What key question helps determine the cause of normocytic anemia?
👉 “How do we know if it’s destruction or underproduction?”
By checking the reticulocyte count:
🔼 High = destruction (marrow is responding)
🔽 Low = underproduction (marrow is failing)
Reticulocyte Count | Meaning | Examples |
|---|---|---|
🔼 High (>3%) | Bone marrow responding → Hemolysis or blood loss | Sickle cell, G6PD deficiency, PNH, IHA |
🔽 Low (<1%) | Bone marrow not responding → Underproduction | Chronic kidney disease (↓EPO), Aplastic anemia, Marrow suppression |
What are reticulocytes?
Young red blood cells (RBCs) recently released from the bone marrow. On a smear, they appear larger and bluish due to residual RNA.
What is the normal reticulocyte count in blood?
1–2% of total RBCs.
What is the lifespan of a red blood cell (RBC)?
What percentage of RBCs are normally replaced daily by new reticulocytes?
About 120 days; Approximately 1% of RBCs per day
How does the bone marrow respond to anemia?
By increasing reticulocyte production to replace lost RBCs.
What does a reticulocyte index (RI) >3% indicate?
A good bone marrow response, suggesting RBC destruction (hemolysis) or blood loss.
What does a reticulocyte index (RI) <3% indicate?
Bone marrow underproduction, suggesting an issue with RBC synthesis (e.g., chronic disease, marrow failure).
What is peripheral destruction divided into?
Two categories
Extravascular OR Intravascular hemolysis
What system is responsible for RBC destruction in extravascular hemolysis?
🧠 The reticuloendothelial system — primarily macrophages in the liver, spleen, and lymph nodes
What happens to RBCs during extravascular hemolysis?
🔬 Macrophages consume RBCs and break down hemoglobin into heme and globin
What does heme break down into?💥 Heme → iron + bilirubin (⚠ not protoporphyrin)
What does globin break down into?
💪 Globin → amino acids, which can be reused by the body
What are the main symptoms/signs of extravascular hemolysis?
Anemia
Splenomegaly
Jaundice
Increased risk of bilirubin gallstones
Why does splenomegaly occur in extravascular hemolysis?
Due to hypertrophy of splenic macrophages actively destroying abnormal or aged red blood cells (RBCs).
What causes jaundice in extravascular hemolysis?
Breakdown of heme from RBCs → unconjugated bilirubin (BR) → builds up in blood → jaundice
Why is there an increased risk of bilirubin gallstones?
Chronic hemolysis—> excess bilirubin excretion into bile → forms pigment gallstones.
What lab finding indicates compensatory bone marrow activity?
Marrow hyperplasia with reticulocyte index (RI) > 3%
What happens to heme after RBC destruction?
Old RBCs break → make bilirubin
1⃣ Old red blood cells break down
2⃣ They release heme
3⃣ Heme becomes unconjugated bilirubin (not water-soluble)
➡ This bilirubin travels to the liver
4⃣ The liver conjugates it = makes it water-soluble
➡ Now it can leave the body
5⃣ It goes into bile → small intestine
6⃣ Gut bacteria change it to urobilinogen
Then it splits:
✅ Stool → becomes stercobilin → makes poop brown
✅ Urine → becomes urobilin → makes pee yellow
Is bilirubin in extravascular hemolysis conjugated or unconjugated?
Unconjugated bilirubin, since it’s released from RBC breakdown before liver conjugation.
NOTES: 🧬 What “Conjugated” Means
Conjugated bilirubin = bilirubin that has been processed (conjugated) by the liver.
The liver adds glucuronic acid to unconjugated bilirubin, making it water-soluble.
→ This allows it to be excreted into bile and then into the intestine.
🧪 Think:
“Conjugated = combined with glucuronic acid = can dissolve in water = can leave the body.”
🧫 Unconjugated Bilirubin
Formed before the liver processes it.
It’s fat-soluble, not water-soluble.
Travels in the blood bound to albumin to reach the liver for conjugation.
🩸 In Extravascular Hemolysis
RBCs are destroyed outside blood vessels (mainly in the spleen and liver macrophages).
This releases heme → unconjugated bilirubin.
So, the bilirubin before reaching the liver is unconjugated.
✅ Answer:
In extravascular hemolysis, bilirubin is unconjugated (indirect).
What is intravascular hemolysis?
RBC destruction that occurs within blood vessels (rather than in the spleen or liver as in extravascular hemolysis).
What are the main symptoms and signs of intravascular hemolysis?
Anemia
Jaundice (due to ↑ unconjugated bilirubin)
Increased risk of bilirubin gallstones
What are the key lab findings in intravascular hemolysis?
Hemoglobinemia: free hemoglobin in plasma
Hemoglobinuria: hemoglobin in urine (dark or red urine)
Hemosiderinuria: renal tubular cells absorb iron from hemoglobin → shed hemosiderin in urine
Why does hemoglobinemia occur?
Free hemoglobin is released directly into circulation when RBCs lyse inside vessels.
Why does hemoglobinuria occur?
The kidneys filter excess free hemoglobin → it appears in urine, giving it a red or cola color.
Why does hemosiderinuria occur later on?
Renal tubular cells absorb hemoglobin’s iron → store it as hemosiderin → slough off into urine after cell death.
What type of bilirubin increases in intravascular hemolysis?
Unconjugated (indirect) bilirubin, since it comes from RBC breakdown before liver conjugation.
🩸 Hereditary Spherocytosis
What is the underlying cause of hereditary spherocytosis?
Which RBC membrane proteins are defective in hereditary spherocytosis?
An inherited defect of RBC membrane tethering proteins (Spectrin, ankyrin, and band 3.1) —> RBCs to lose their shape (more spherical)—> destroyed via macrophages
What structural change occurs in RBCs due to this defect?
Formation of membrane blebs and gradual loss of surface area, producing spherical RBCs (spherocytes).
Why do spherocytes get destroyed?
They lose flexibility and become trapped in the spleen, where they’re phagocytosed by splenic macrophages, leading to anemia
🔬 Clinical and Lab Findings:
What are the main symptoms/signs of hereditary spherocytosis?
Splenomegaly
Jaundice (↑ unconjugated bilirubin)
↑ risk of bilirubin gallstones
↑ risk of aplastic crisis with parvovirus B19 infection of erythroid precursors
What lab findings are characteristic of Hereditary Spherocytosis?
Spherocytes (loss of central pallor)
↑ RDW (variation in RBC size)
↑ MCHC (mean corpuscular hemoglobin concentration)
What diagnostic test confirms hereditary spherocytosis?
Osmotic fragility test → spherocytes lyse in hypotonic (low osmotic) solution.
What is the treatment for hereditary spherocytosis?
Splenectomy (removes the site of RBC destruction and resolves anemia).
After splenectomy, do you still see spherocytes on the smear?
What additional finding may appear on peripheral smear after splenectomy?
✅ Yes, spherocytes persist (defect is intrinsic to the RBC),
but anemia and jaundice improve because RBCs are no longer destroyed in the spleen.
**Howell–Jolly bodies — nuclear remnants in RBCs (normally removed by the spleen).
🧬 Howell–Jolly Bodies – Explained Clearly
💡Definition
Howell–Jolly bodies are small, round, dark purple nuclear remnants (DNA fragments) found inside red blood cells (RBCs) on a peripheral blood smear.
🧠Why They Appear
Normally, the spleen removes these nuclear remnants when filtering blood.
➡ So if you see Howell–Jolly bodies, it means the spleen isn’t functioning or has been removed.
⚕Common Causes
Post-splenectomy 🩺
Functional asplenia (e.g., sickle cell disease → autosplenectomy)
Severe hemolytic anemia (if splenic clearance is overwhelmed)
🔬Appearance on Smear
Small, single, round inclusions (usually one per RBC)
Dark blue or purple with Wright–Giemsa stain
Located inside the RBC (not on the surface)
📍Mnemonic
“H for Howell–Jolly = H for Hypo-spleen”
If the spleen is missing or malfunctioning → Howell–Jolly bodies appear.
🧬 Sickle Cell Anemia
What is the genetic cause of sickle cell disease?
An autosomal recessive (AR) mutation in the β-chain of hemoglobin.
Val replaces Glu at β-6 — sickles when de-oxygen-ated.
What is the difference between sickle cell disease and sickle cell trait?
Sickle cell disease: Two abnormal β genes → ~90% HbS
Sickle cell trait: One abnormal β gene → ~45% HbS, remaining is normal HbA
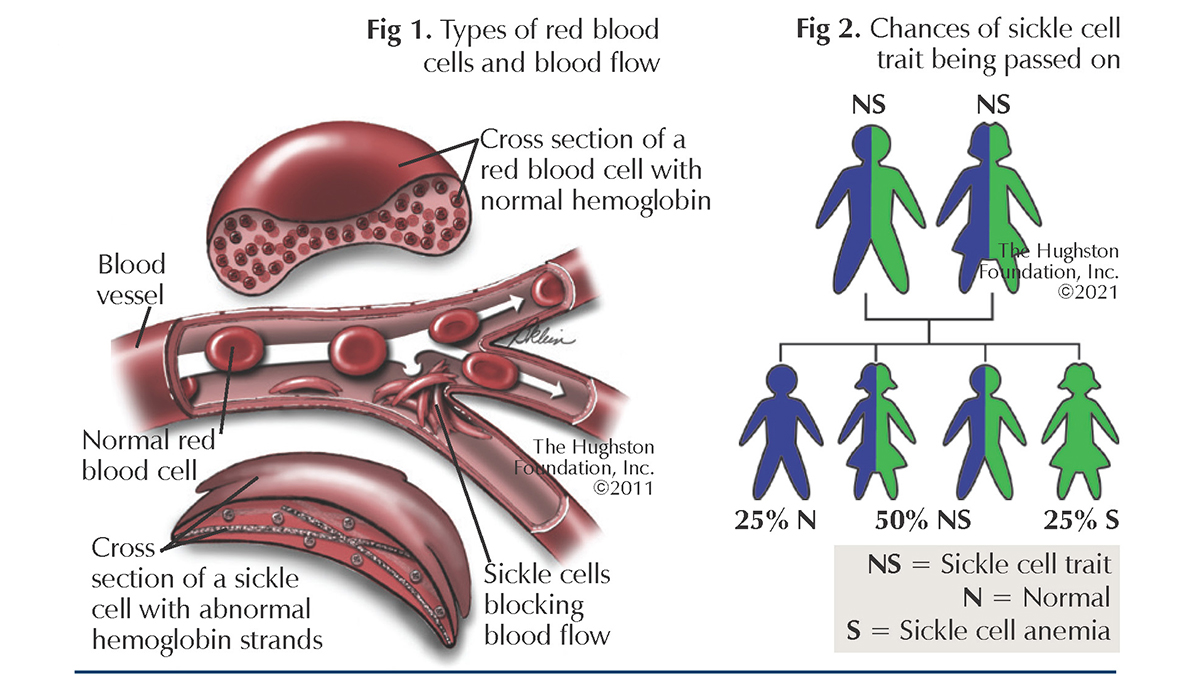
What is HbS?
HbS is the mutant hemoglobin that polymerizes (sticks together) under stress — when blood is deoxygenated, acidic, or dehydrated — forming long needle-like fibers inside RBCs.
Under what 3 conditions does HbS polymerize most easily?
➡ Low oxygen (hypoxia)
➡ Dehydration
➡ Acidosis (low pH)
➡Altitude
🩸 These factors promote deoxy-HbS polymerization, causing RBC sickling.
NOTE: Polymerization is reversible → damages RBC membrane
When oxygen returns (reoxygenation), the sickle shape can reverse, but:
Repeated cycles of sickling and unsickling damage the RBC membrane,
making the cell more fragile and prone to hemolysis (destruction).
Over time, these cells become permanently deformed and are removed by the spleen.
What happens to RBCs during polymerization?
HbS fibers distort RBC shape → “sickle” or crescent-shaped.
Sickled cells are rigid and sticky, blocking small vessels.
Sickling is initially reversible with reoxygenation, but repeated episodes cause membrane damage, making sickling irreversible.
What does repeated sickling cause over time?
Membrane damage → rigid cells
Hemolysis (RBC destruction)
Vaso-occlusion (clogging of small vessels)
What type of hemolysis occurs in sickle cell disease — extravascular or intravascular?
Primarily extravascular hemolysis (RBCs destroyed by splenic macrophages).
Some intravascular hemolysis also occurs from mechanical membrane rupture of fragile sickled RBCs.
🧪 → Leads to anemia, jaundice, and ↑ bilirubin gallstone risk.
Why does sickle cell disease cause anemia?
Because the lifespan of RBCs decreases from ~120 days to ~20 days due to recurrent destruction
What long-term effect does repeated sickling have on the spleen?
Chronic vaso-occlusion and infarction → autosplenectomy (fibrotic, shrunken spleen).
➡ Leads to functional asplenia → ↑ risk of infection by encapsulated organisms
(Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis)
What key lab findings reflect hemolysis in sickle cell disease?
↑ Reticulocyte count
↑ LDH
↑ Unconjugated bilirubin
↓ Haptoglobin (binds free Hb in intravascular hemolysis)
How does sickle cell disease differ pathophysiologically from thalassemia?
Sickle cell: Structural defect in Hb → abnormal shape & polymerization
Thalassemia: Quantitative defect → ↓ globin chain production
What is a vaso-occlusive crisis?
A painful episode caused by blockage (occlusion) of small blood vessels due to sickled, rigid RBCs that obstruct blood flow → leads to ischemia and tissue infarction.
🧠 Sickle Cell Anemia – Clinical Findings
What type of hemolysis occurs in sickle cell disease?
➡ Both extravascular and intravascular hemolysis, but extravascular predominates.
Damaged, sickled RBCs are mainly destroyed by macrophages in the spleen.
What is extravascular hemolysis, and what causes it here?
The reticuloendothelial system (RES)—especially the spleen—removes RBCs with damaged membranes.
These RBCs are trapped and phagocytosed.
Leads to anemia, jaundice (↑ unconjugated bilirubin), and pigment (bilirubin) gallstones.