Gen Bio Final Practice Quizzes
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
88 Terms
The smallest particle into which an element can be divided and yet still display the properties of that element is the:
atom
Substances that cannot be broken down to other substances by ordinary chemical means are termed:
elements
Which can be shown to be INCORRECT?
I. Hypothesis
II. Theory
III. Prediction
I, II, and III
Sequence chemical synapse operation. An action potential arrives at a synaptic terminal. What happens next?
The action potential ceases to exist.
Sequence scientific inquiry: which step comes first?
making a hypothesis
When glucagon is received by a liver cell, the cell:
uses glycogen to make glucose.
To be considered life, an object must:
I. contain cells
II. have water
III. contain carbon
IV. contain DNA
None of these are needed for something to be considered life.
Which of the following elements have the same number of electrons in the first energy level? (atomic number Element)
2He 4Be 9F 10Ne 12Mg 20Ca
2He, 4Be, 9F, 10Ne, 12Mg, and 20Ca
In atoms, a p orbital can hold a maximum of ______ electrons.
2
What is the maximum number of electrons in the 4th shell of an atom?
32
Compare and contrast alpha and beta cells.
Both are found in the pancreas.
The monomers for DNA and RNA are?
nucleotides
To be considered life, an object must:
I. contain cells
II. have water
III. contain carbon
IV. contain DNA
None of these are needed for something to be considered life.
13Al would have ______ electrons in its valence shell? (atomic number Element)
3
20Ca would be found in the same Group on the periodic table as which of the following? (atomic number Element)
2He 5B 9F 12Mg 15P
12Mg only
Sequence glucose homeostasis. Insulin targets the ____________________ while glucagon targets the ___________________.
liver and skeletal muscle; liver and skeletal muscle
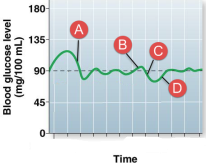
Glycogen stores would be increasing at:
Point A
In atoms, a p orbital can hold a maximum of ______ electrons.
2
In the Periodic Table of the Elements, elements in the same Group have the same number of:
e- in the valence shell.
A covalent bond forms when atoms share ____ pair(s) of electron(s).
1
Learning is:
using memories to improve.
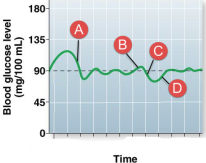
Sequence glucose homeostasis. Insulin levels would be high at:
A
Which type of bond connects an oxygen atom and a hydrogen in a water molecule?
polar covalent
The two carbons in ethyne (C2H2) are connected to each other by a _____________.
triple covalent bond
In atoms, the 2p subshell can hold a maximum of ______ electrons while the 3p subshell can hold a maximum of ________electrons.
6; 6
Sequence the scientific method. After doing background research, one next:
creates a hypothesis.
What is the electrical charge of this molecule: H2
0
9F would have ______ electrons in its valence shell? (atomic number Element)
7
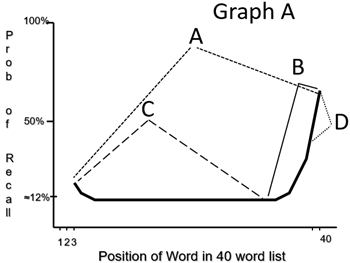
In Graph A, which letter best indicates those words which are stored in the long-term memory?
C
In atoms, an s orbital can hold a maximum of ______ electrons while a p orbital can hold a maximum of ______ electrons.
2; 2
Water (H2O) is polar because it is constructed using __________ bonds.
polar covalent
Compare and Contrast prediction and hypothesis. Which statement is TRUE?
Predictions must logically flow from the hypothesis.
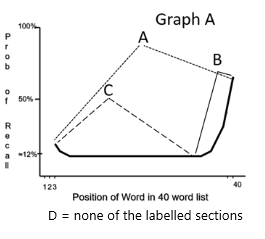
In Graph A, which letter best indicates the words stored in the sensory memory?
D
In atoms, the first energy level can hold a maximum of ______ electrons while the second energy level can hold a maximum of ___________ electrons.
2; 8
Water (H2O), a polar molecule, has what electrical charge?
0
14Si has an atomic mass of 28. Thus, you know it has:
14 protons, 14 electrons, and 14 neutrons.
The Pauling Scale indicates that H has an electronegativity of 2.1. Knowing this value, you can predict that H2 will have a(n) _______________ bond.
non-polar covalent
For a hydrogen bond to form, there must be:
polar covalent bonds in the participating molecules.
Which is an example of a Component-Level Outcome?
Identify (ID)
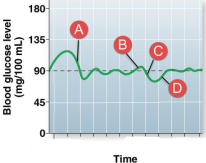
Sequence glucose homeostasis. Glucagon levels would be high at:
D
Compare and contrast functional groups. Which act as an acid (H+ donor)?
I – carbonyl
II – hydroxyl
III – methyl
IV - phosphate
IV only
Which type of bond connects an O and an H in a water molecule?
polar covalent
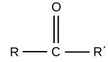
This functional group allows one to identify this molecule as a:
ketone.
In atoms, the first energy level can have up to __________ orbitals.
1
Identify the molecules that are hydrophilic:
I. oils
II. NaCl
III. table sugar
II and III
Which are polymers of glucose?
cellulose, glycogen, and starch
In atoms, a p orbital can hold a maximum of ______ electrons.
2
20Ca++ would have ______ electrons in its valence shell? (atomic number Element)
8
A patient is given a drug that inactivates glucagon. As a result, the patient will NOT be able to:
increase blood glucose levels after not eating for several hours.
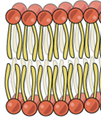
The heads of the molecules below contain which functional group?
phosphate
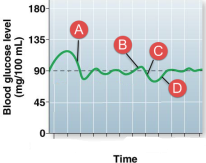
Sequence glucose homeostasis. Glucagon levels would be high at:
D
What is the maximum number of electrons in the 4th shell of an atom?
32
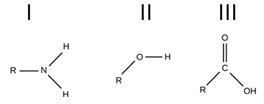
Which of functional groups allow you to definitely identify a molecule as an amino acid?
I and III only
Sequence chemical synapse operation. An action potential arrives at a synaptic terminal. What happens next?
The action potential ceases to exist.
To create a polypeptide out of two amino acids we would need to do ____________.
a dehydration synthesis
To be considered life, an object must:
I. be cellular.
II. be able to reproduce.
III. contain nucleic acids.
IV. have order.
II and IV only
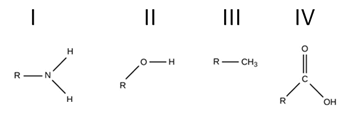
Which of the following functional groups are hydrophilic?
I, II, and IV
Identify which type of biological molecule this is.
fatty acid
Compare and Contrast prediction and hypothesis. Which statement is TRUE?
Predictions must logically flow from the hypothesis.
In proteins, the alpha helix is an example of:
secondary structure.
20Ca++ would have ______ electrons in its valence shell? (atomic number Element)
8
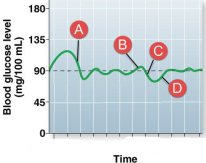
At which point in time would beta cells be most active?
A
Water, a polar molecule, has an electrical charge of:
0
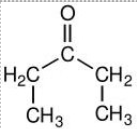
The functional groups in this molecule allow one to identify it as a(n):
ketone.
Chitin is a ______________________ and characteristic of _______________________.
carbohydrate; insects
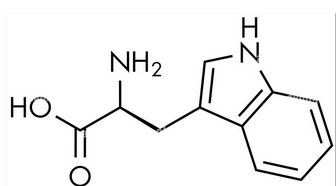
This molecule is a:
amino acid.
Identify in which phenomena(on) hydrogen bonding is an important factor.
I. Water's cohesiveness
II. Movement of water in plant xylem
III. Capillary action
IV. Primary structure of proteins
I, II, and III
In atoms, the first energy level can have up to __________ orbitals.
1
The linear sequence of amino acids in a protein is the protein's:
primary structure.
Learning is:
using memories to improve.
In amino acids, the R group is bound to the ______________.
alpha C
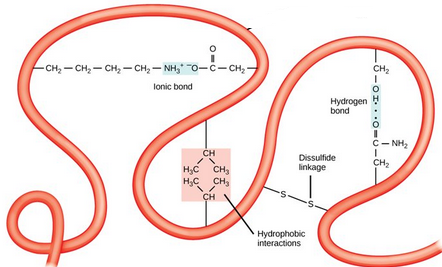
This molecule shows:
the tertiary structure of a polypeptide
Identify which is/are a type of covalent bond:
I. ester
II. hydrogen
III. glycosidic
IV. peptide
I, III, and IV
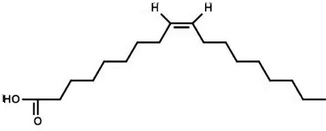
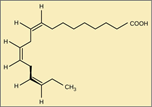
This molecule is a:
an unsaturated fatty acid
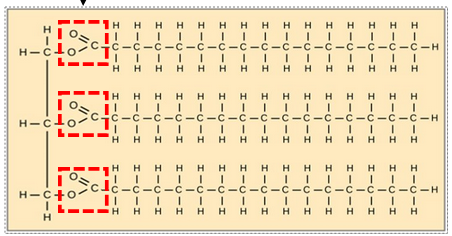
Identify the type of bond indicated in the dashed boxes.
Ester
Identify which is(are) TRUE of amino acids.
I. Can act as a base.
II. Can act as an acid.
III. Can be involved in a reaction catalyzed by a dehydrogenase.
IV. The overall behavior of a particular amino acid is determined by the R group.
I, II, III, and IV
20Ca would be found in the same Group on the periodic table as which of the following? (atomic number Element)
2He 5B 9F 12Mg 15P
12Mg only
When glucagon is received by a liver cell, the cell:
uses glycogen to make glucose.

Identify this molecule; it most likely is:
a polysaccharide.
Compare and contrast a living thing with a non-living thing. Which of the following is required for something to be considered “life”?
Neither cells, carbon, nor water are required.
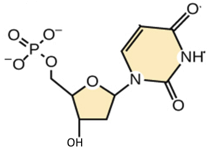
In this molecule, the phosphate group is bound to the ___________ carbon.
5’
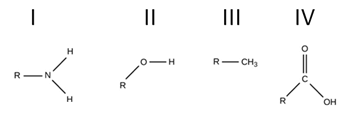
Which of the following functional groups is/are hydrophobic?
III only
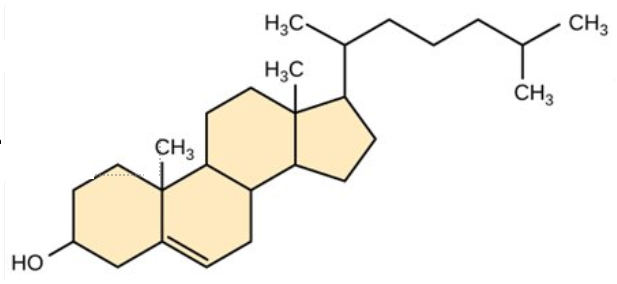
This molecule is a:
Steroid.
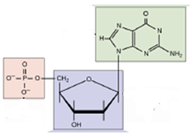
Identify this molecule; it is a(n):
nucleotide
The monomers for DNA and RNA are __________
nucleotides
In atoms, the first energy level can hold a maximum of ______ electrons while the second energy level can hold a maximum of ___________ electrons.
2; 8
Acids are:
proton donors.
To create a disaccharide out of two monosaccharides we would need to do ____________ to form a ____________________ bond.
dehydration synthesis; glycosidic