IHS 3400 Midterm Flashcards
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Types of medical settings
ASC (Ambulatory Surgery Center): A modern healthcare facility where patients receive same-day surgical care. They don’t need an overnight hospital stay. It is designed to be less complex, minimally invasive, and low risk, allowing for faster recovery and lower costs.
Hospital Inpatient
Hospital Outpatient
Health Systems
Inpatient Vs. Outpatient
Review and memorize the chart.
Benefits of minimally invasiveness
There is reduced trauma to the body
Faster recovery times
Lower risk of complications
Less pain and discomfort
Improved cosmetic outcomes
Cost-effectiveness: This is due to shorter hospital stays, fewer complications, reduced need for follow-up care.
The longer a surgery continues there is an increase in risk. For every 15 minutes there is a 13% increased risk, every 30 minutes 17% increase, every 60 minutes 37% increase.
Importance of administrators
They ensure that the entire system runs smoothly, efficiently, and ethically. Administrators manage the day-to-day operations of hospitals, clinics, and health systems. They manage the finances, ensure there is regulatory compliance and risk management, perform strategic planning and innovation, coordinate disaster preparedness, and manage crisis and emergency responses.
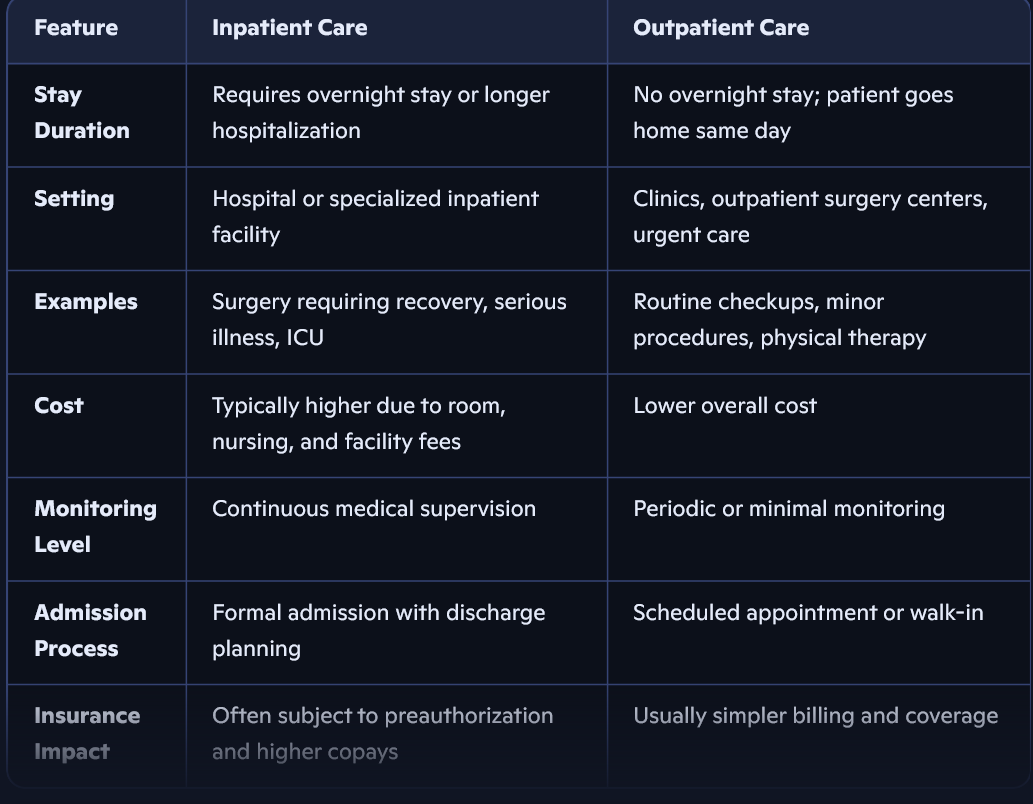
What device is this?
Speed Bridge
Arthrex mission
Helping surgeons treat their patients better.
Medical Education
Benefits of the 3D CADaver:
Consistent anatomy
Pathology replication
Help alleviate the lack of cadaver supplies
Ensure the safety of nearby ongoers since cadavers undergo continuous x-ray and this causes x-ray radiation exposure to those not protected, so using the 3D CADavers improved the safety of others.
Cost-effective
510(k)
510(k):
A premarket notification.
You need to have a predicate device to get a 510(k). A predicate device is a device that is already marketed in the US and can use that comparison for the new device.
You must provide proof that your device is substantially equivalent to the predicate device. Meaning it must have the same intended use and have similar technology.
If the device wasn’t substantially equivalent, then the device would be placed in the class 3 category. If it was substantially equivalent, then the device would have the same class as the predicate.
For a 510(k) it usually takes the FDA 90 days to approve/deny the 510(k).
FDA
Food and Drug Administration
They regulate products like food, drugs, medical devices, vaccines and biologics, cosmetics, tobacco products, and dietary supplements.
Design/Quality/Regulation Phase
Phase 1: Concept
What problem/need are we trying to solve?
Phase 2: Planning
Where, when, how, with whom?
Phase 3: Design
Which inputs will lead to the desired outputs?
Phase 4: Validation
Are we sure?
Phase 5: Launch
Do we consistently provide high-quality products?
Phase 6: Post-market
How do we monitor issues and trends?
Anatomical direction terms
Directional Terms in Anatomy
Superior
Inferior
Posterior
Anterior
Lateral
Medial
Median Sagittal Plane
Transverse plane
Coronal (Frontal Plane)
Sagittal Plane
Supine
Prone
Dorsal Surface
Plantar Surface
Volar
Dorsal
Proximal
Distal
Adduction
Abduction
Eversion
Inversion
Flexion
Extension
Supination
Pronation
Extension
Hyperextension
Flexion (Hand)
Dosiflexion
Plantarflexion
Rotation
Circumduction
Protraction
Retraction
Elevation
Depression
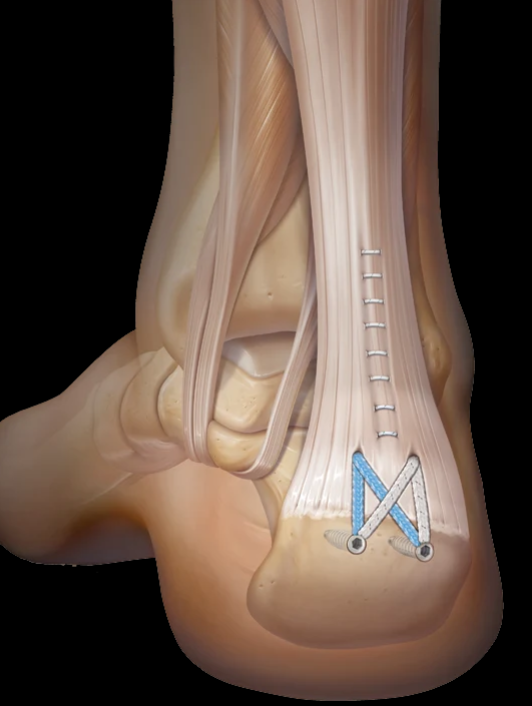
Name the bones of the shoulder
Humerus
Scapula
Clavicle
Glenoid
Acromion
Humeral head
coracoid
How many teams are there in ortho research and what are they?
Operations
Clinical
Biomaterials
Biomechanics
HCP
Health care providers
ASC
Ambulatory surgery center
A modern healthcare facility where patients receive same-day surgical care. They do not need an overnight hospital stay. ASC are designed to be less complex, minimally invasive, and low-risk, allowing for faster recovery and lower costs.
Relationship tiers:
Strategic partner: long-term relationship, the most far-reaching, the best of the best.
Preferred supplies: strong relationship, earns the right to seeling opportunities because of performance and capabilities.
Approved supplier: seen as a transactional vendor, not emotionally connected, fundamental lack of respect and appreciation of value delivered.
Class categories
Class I General Instruments
Exempt from FDA submission
Ex. Saw blades, scalpel
74% of the device are exempt from doing a premarket notification process.
Class II
Typically requires a 510(k)
Ex. Rods, screws, suture, bone anchors, PRP devices
Class III (PMA or BLA)
Clinical data required
Ex. Synojoyny-hyaluronic acid
A premarket approval (PMA) will be required unless there is already a similar device on the market that hasn’t been called for. If that happens, then that device will do a 510k instead.
For a PMA it usually takes 180-320 days for the FDA to approve.
For a PMA, the benefits of the device must outweigh the cons, the device can’t rely on another device so it has to be independent, and it must benefit a vast majority of the target audience.
Clinical research history and ethics
1947: Nuremberg Code- It was made in response to atrocities committed to prisoners by Nazis. The foundation of research is to protect the subjects’ rights in medical research.
1964: Declaration of Helsinki- Patient’s health is always the doctor’s first consideration. Key element introduced: protection of vulnerable groups.
1976: Medical Device Amendments- Provided reasonable assurance of the safety and effectiveness of medical devices before they get to the market. Established 2 pathways for devices: PMA and 510(k).
1979: Belmont Report- Basic ethical principals for conducting research that involves human subjects. Written in response to the infamous tuskegee syphilis study.
1980: Investigational Device Exemption- Allows investigational device to be used to collect safety data and effectiveness data.
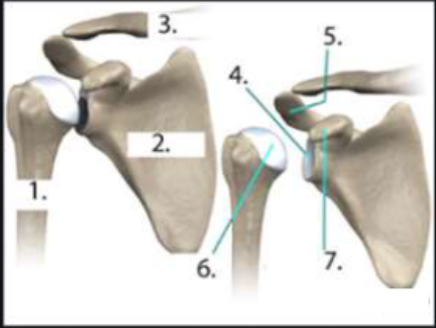
What device is this?
Tightrope
What device is this?
Suture tape

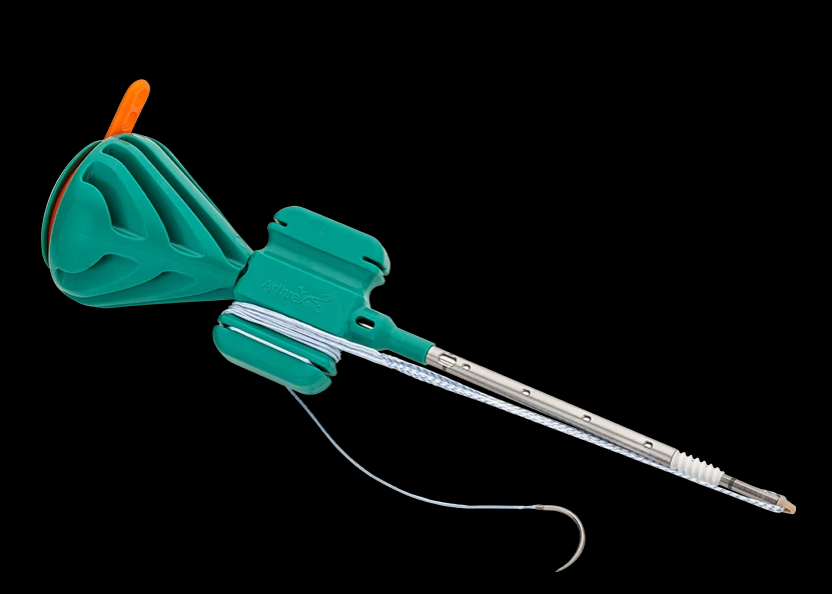
What device is this?
Scorpion
What device is this?
Swivelock anchors
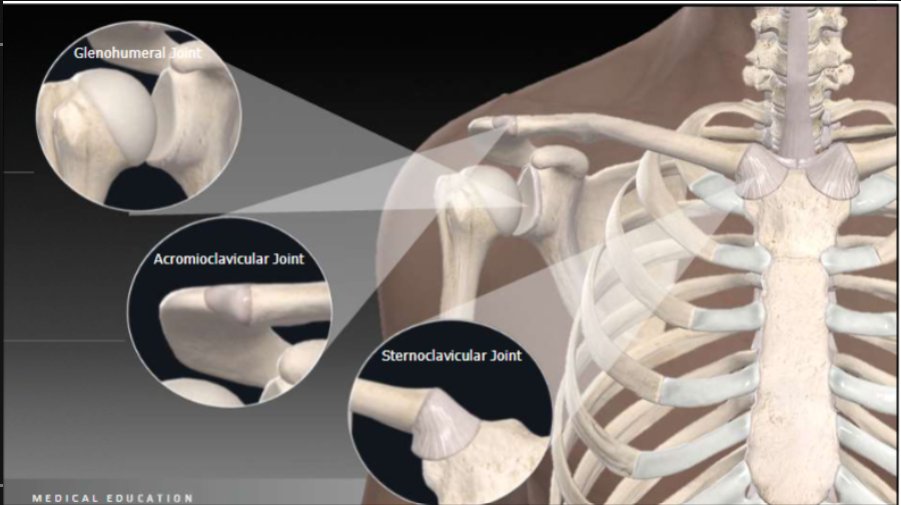
Joints of shoulder
Joints:
Glenohumeral joint (ball & socket)
Acromioclavicular joint (plane joint)
sternoclavicular joint (saddle joint)
Orthopedic research
Technology Consultants
What does a tech consultant do?
Use evidence based info to educate customers on the safe and effective use of our products and procedures and challenge their way of delivering value through improved treatment and patient outcomes. They inspire the adaptation of new tech to help surgeons treat their patients better” -Reinhold Schmieding
Legal Process
Patents and patient law
QARA
Quality Assurance and Regulatory Affairs
Product Lifecycle
Risk increases as the product moves through the phases
Processes at all phases must be defined and documented
Results of studies must be recorded and reported
The company is responsible for its vendors and service providers.
QARA involved in process and product at each phase to ensure that all external and internal requirements are met, helping drive safety, reliability, repeatable outcomes, and continuous improvement.
Phase 1: Concept
Phase 2: Planning
Phase 3: Design
Phase 4: Validation
Phase 5: Launch
Phase 6: Post-market
Quality- All phases
Create a device that can be a solution to an issue from America or worldwide. The challenge/injury/disease can be anything, as long as you describe it in detail of what the challenge is and what the solution/goal is.
Purpose of Quality organization
to implement and maintain systems that ensure reliable, repeatable production for the integrity and preservation of the safety, identity, strength, purity, potency, and quality of the product for its intended use and throughout its useful life.
Purpose of regulatory affairs
to act as an internal and external liaison in order to facilitate the process at all stages of the regulated product lifecycle.
Internally, RA liasises at the interphase of R&D, clinical research, manufacturing, and marketing
Externally: it is the key interface between the compay and the regulatory authorities.