Chem 2.1A Formation of Ions + 2.1B Formation of Ionic Compounds
1/13
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Ionic compounds do not form…
in isolation
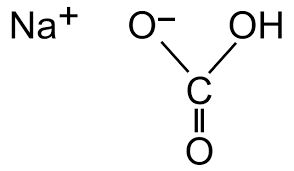
What is Sodium Bicarbonate?
NaHCO₃
What is Ammonium?
NH₄⁺
What is Hydroxide?
OH⁻
What is Nitrate?
NO₃⁻
What is Bicarbonate/Hydrogencarbonate?
HCO₃⁻
What is Carbonate?
CO₃²⁻
What is Sulfate?
Phosphate PO4
SO₄²⁻
What is Phosphate?
PO₄³⁻
What are Electrostatic Forces?
ionic bonds
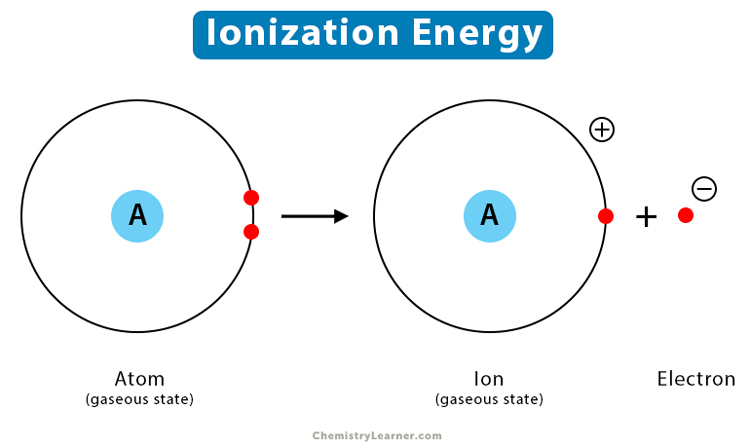
What is ionization and when does it occur?
electrons transferring between atoms
occurs when an atom that loses electrons passes them directly to an atom that gains electrons
What is the formal way to describe what an ionic compound is?
ions held together through electrostatic forces
Ionic compounds are neutral because…
there is no net gain or loss of electrons
Summarize Ionic Compounds (in case Mr. Cory asks for the qualities of one)
not formed in isolation
formed through ionization
held together through electrostatic forces
neutral