Biochem CHEM3040 Smithrud Problems
1/523
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
524 Terms
C
Which is an amine?

D
Which is a carboxylic acid?
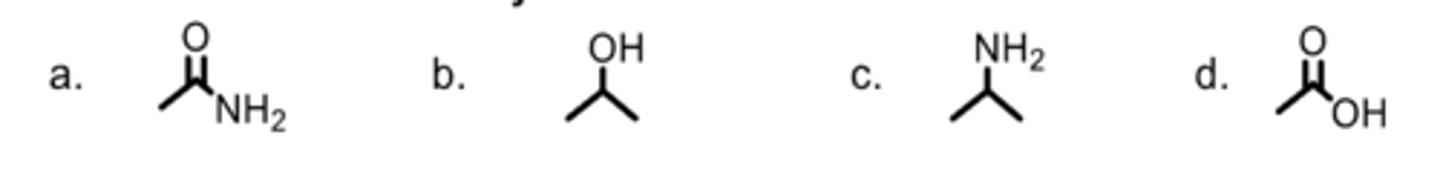
D
Which is an alkyl phosphate?
B
Which is an alcohol functional group?
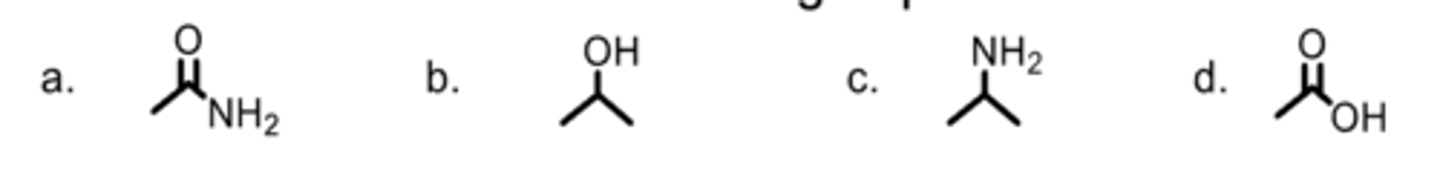
A
Which is an amide functional group?
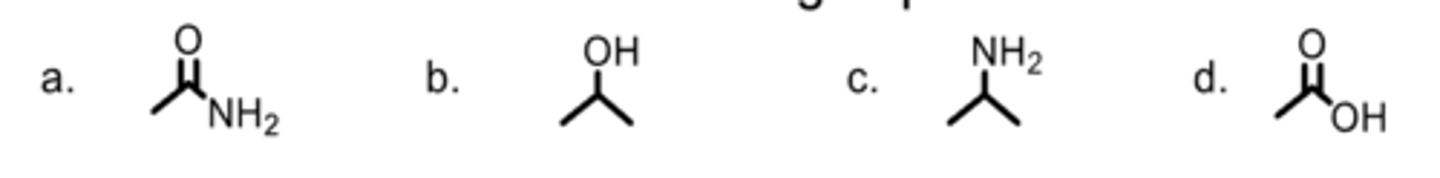
A
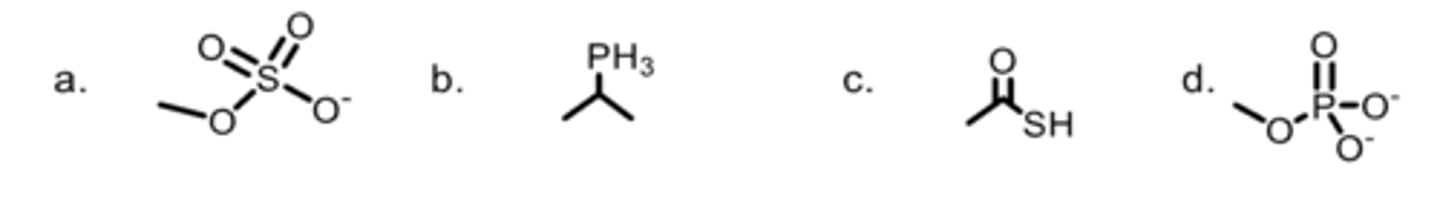
Which is an alkyl sulfate?
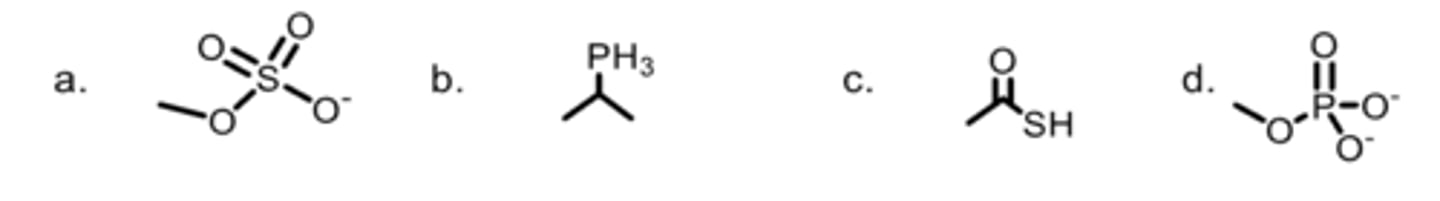
C
Which bond has shared electrons?
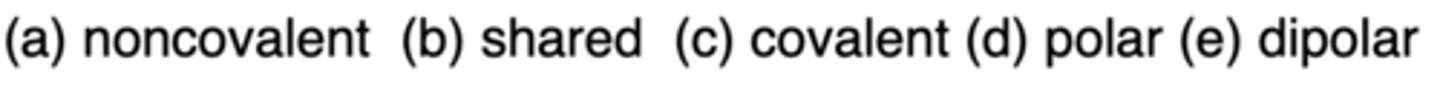
C
Which bond type is not strongly affected by the environment?
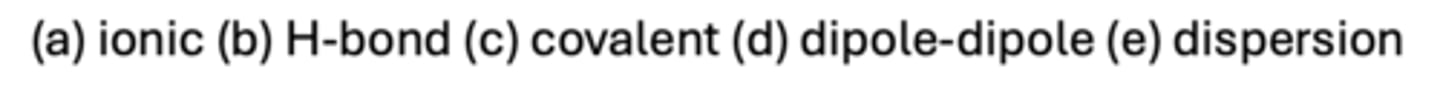
C
Which type of bond does not generally form between proteins and ligands when they bind

A
Which bond gives more possible conformation

E
Rank the length of the bonds from longest to shortest
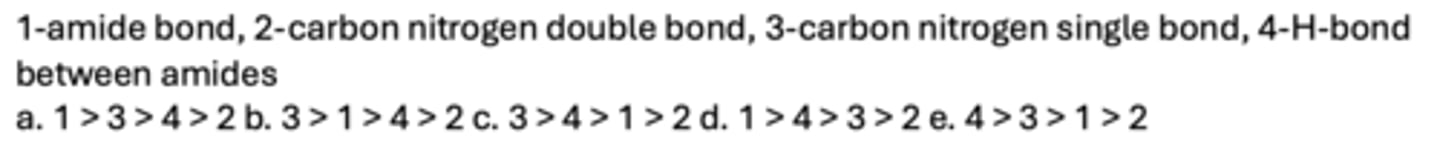
C
Which is the weakest bond in the gas phase?
A. Dipole-Dipole
B. Hydrogen
C. Dispersion
D. Ionic
D
Which is the strongest bond in the gas phase?
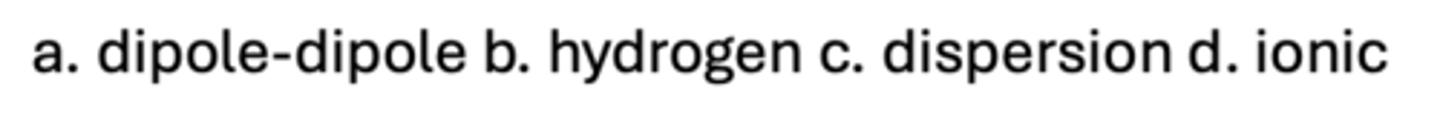
C
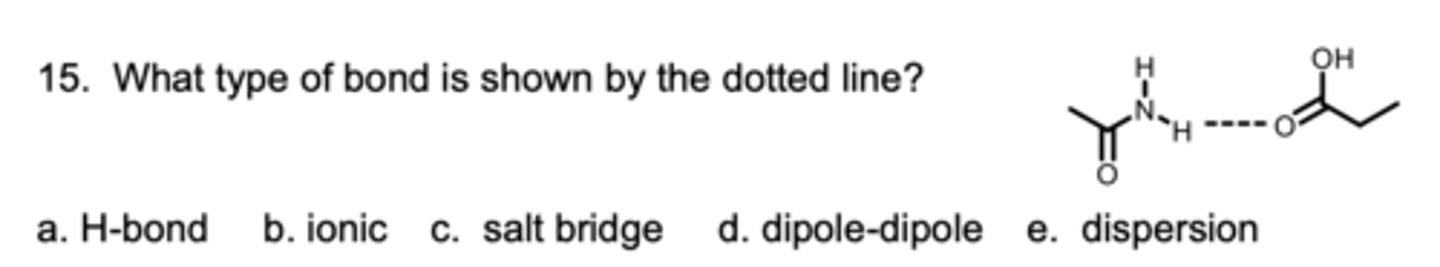
What type of bond is shown by the dotted line?

A
What type of bond is shown by the dotted line?
D
What type of bond is shown by the dotted lines?

E
What type of bond is shown by the dotted line?
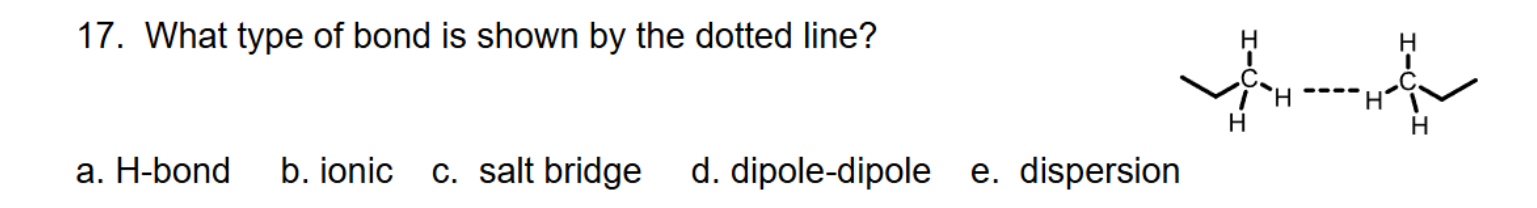
E
What type of bond requires random shifts in e-clouds to form?

D
Rank the compounds from highest to lowest in boiling points.
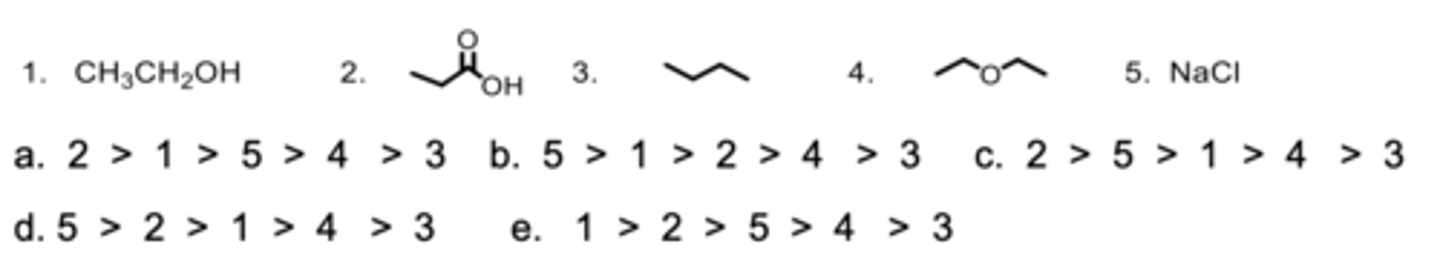
D
Rank the compounds from highest to lowest in boiling points.
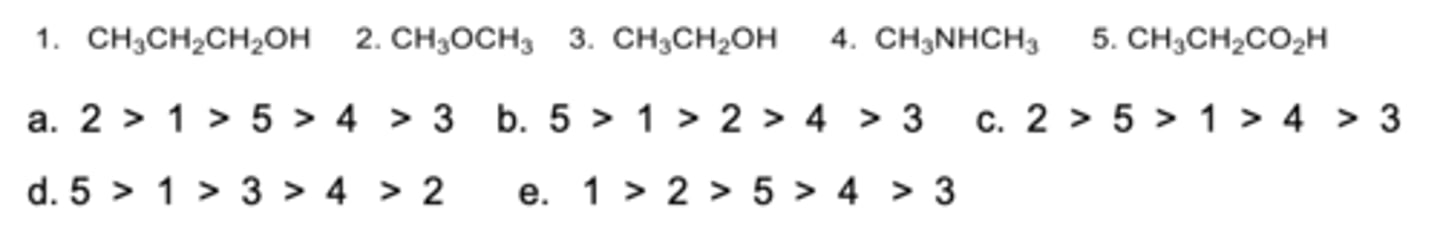
D
Rank the compounds from highest to lowest in boiling points.
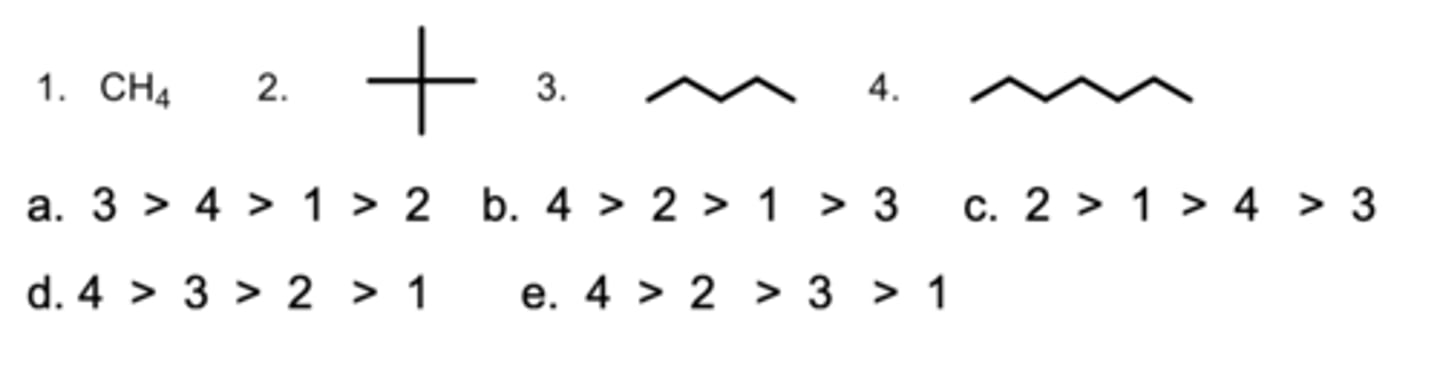
B
Rank the compounds from highest to lowest in boiling points.
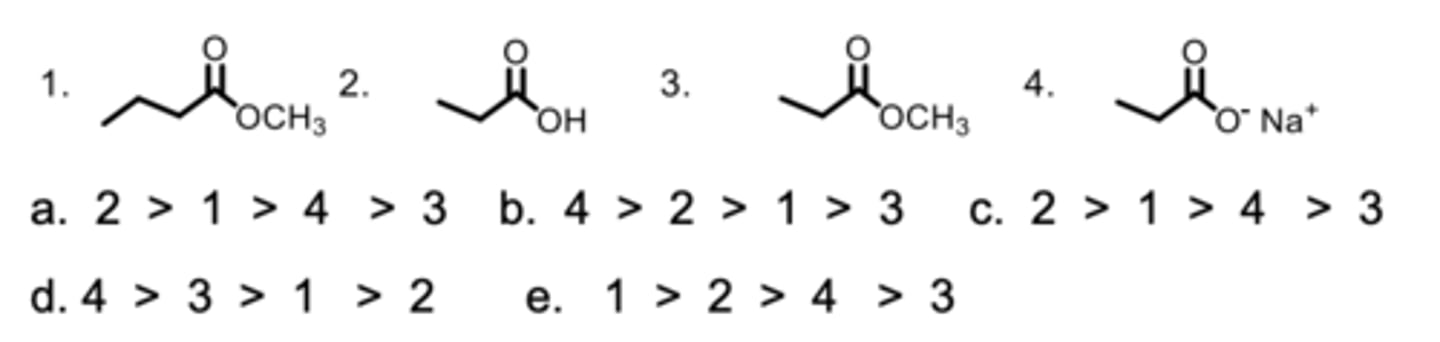
E
Which molecule has the highest boiling point?
B
Rank the molecules from highest to lowest solubility in water.
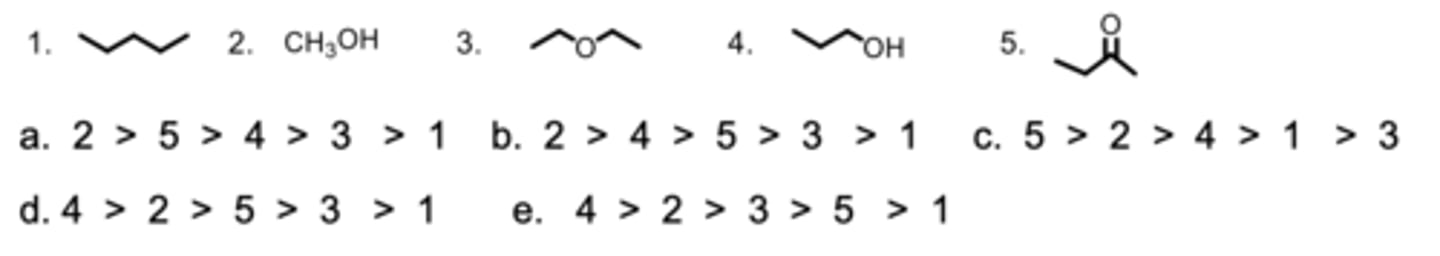
A
Rank the molecules from highest to lowest solubility in hexane.
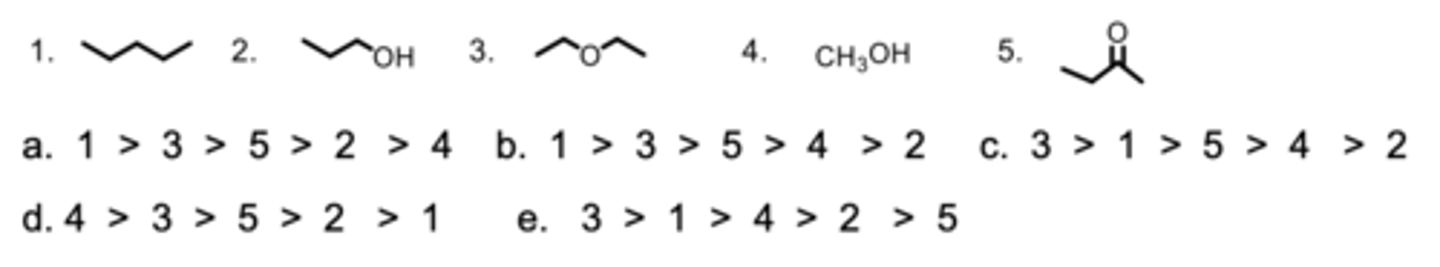
C
Rank the molecules from highest to lowest solubility in water.
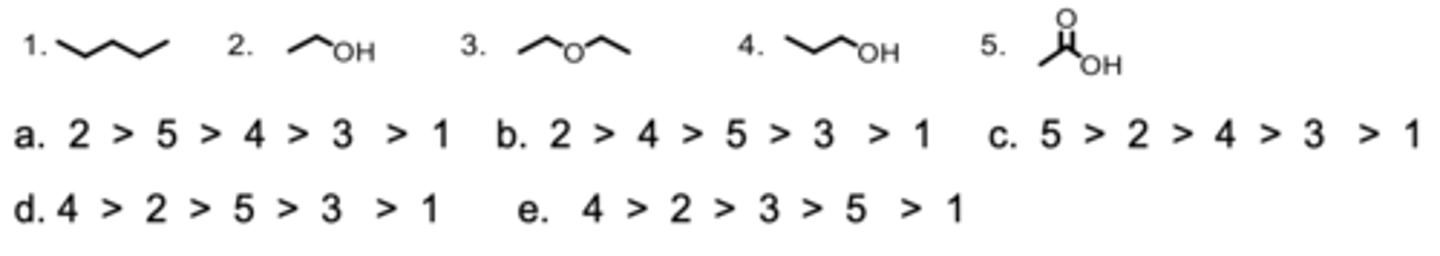
D
Rank the molecules from highest to lowest solubility in hexane.
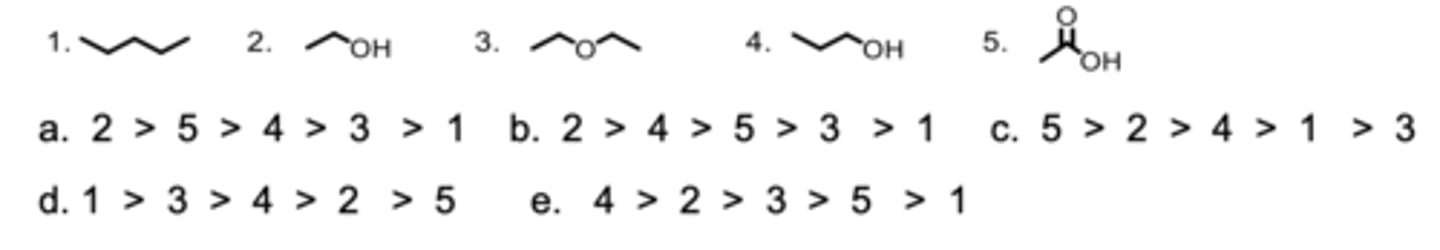
D
Which is more soluble in water: NaCl or ethanol?
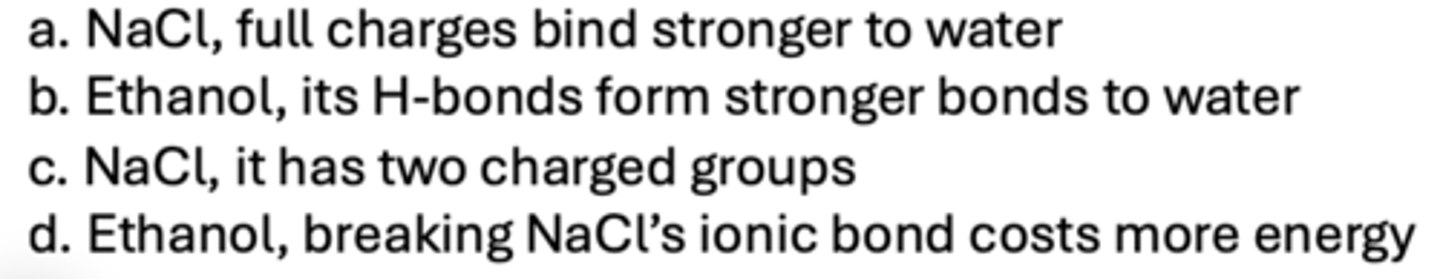
C
Which is more soluble in water: ethanol or acetic acid

B
Which is more soluble in water: ethanol or diethyl ether

A
Which molecule has the most wasted heat, assuming all have the same boiling point?
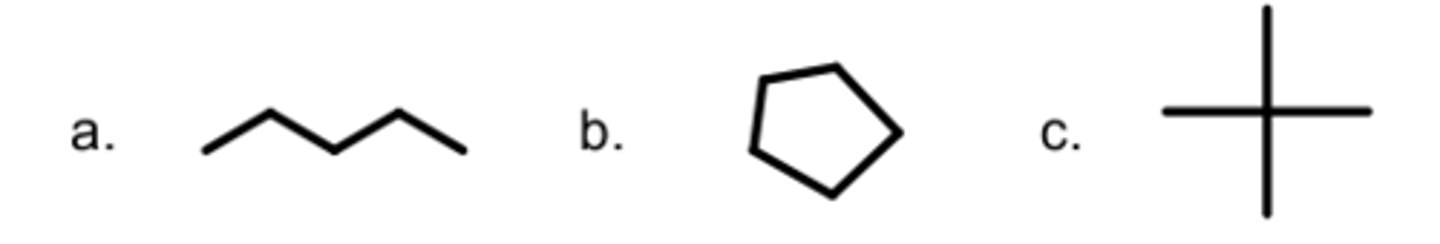
C
Which system has the highest entropy?

A
Which state has the highest entropy?
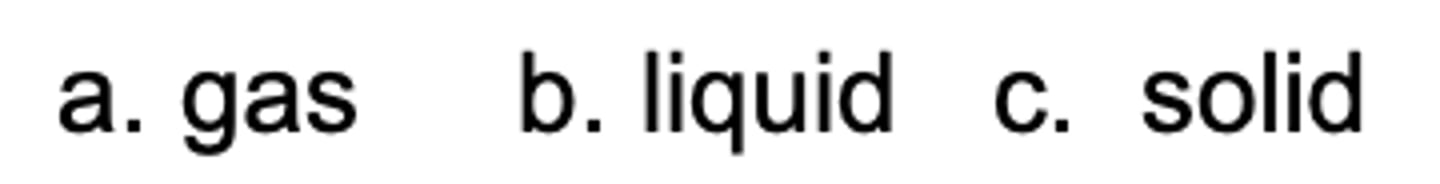
A
Which system has the lowest entropy?

C
Which state has the lowest entropy?
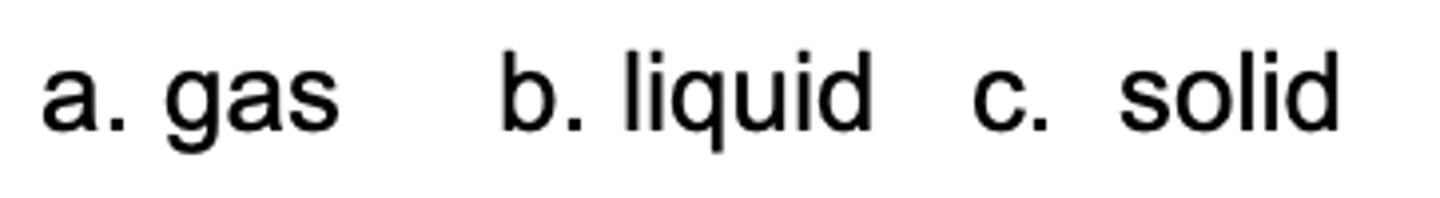
D
The salt bridge formed between ____ would be the most exothermic in?
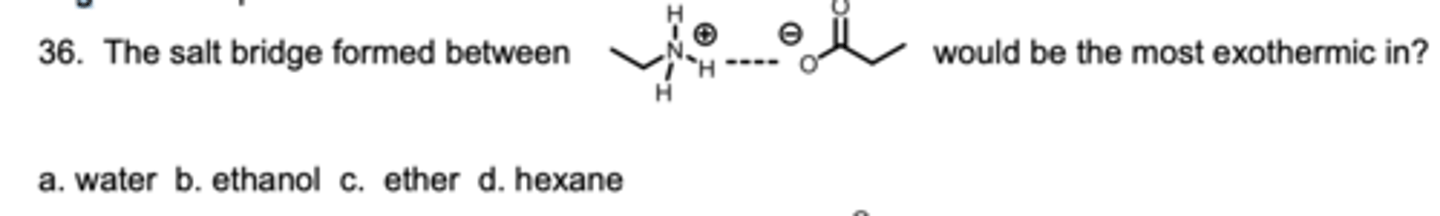
A
The salt bridge formed between _____ would be the least exothermic in?
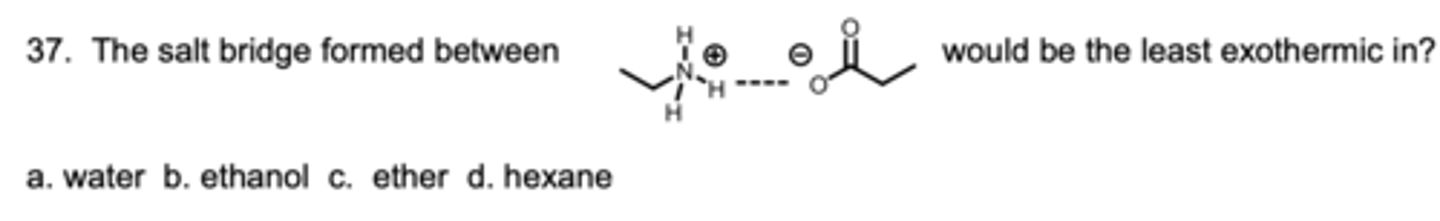
D
Which diagram shows a spontaneous reaction?
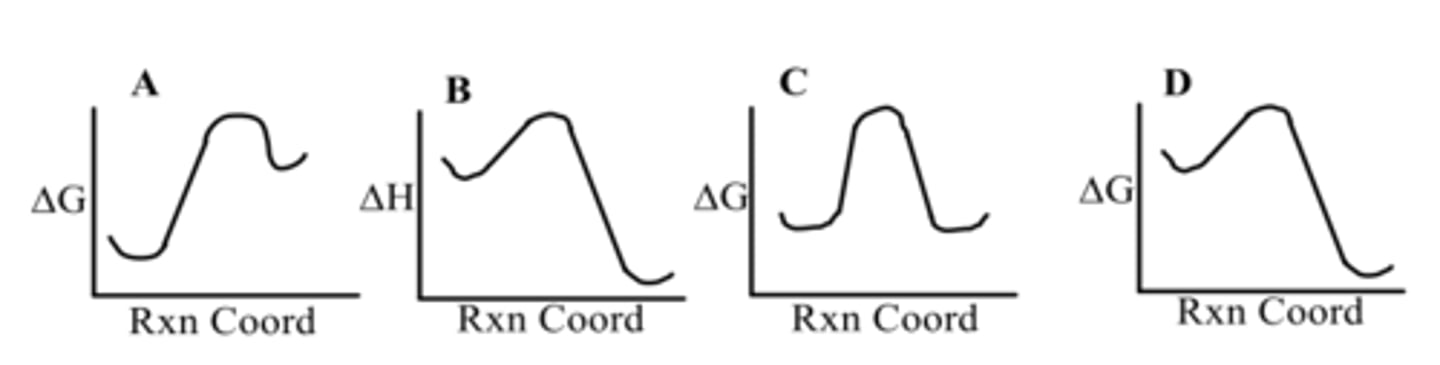
D
Which diagram shows an exergonic reaction?
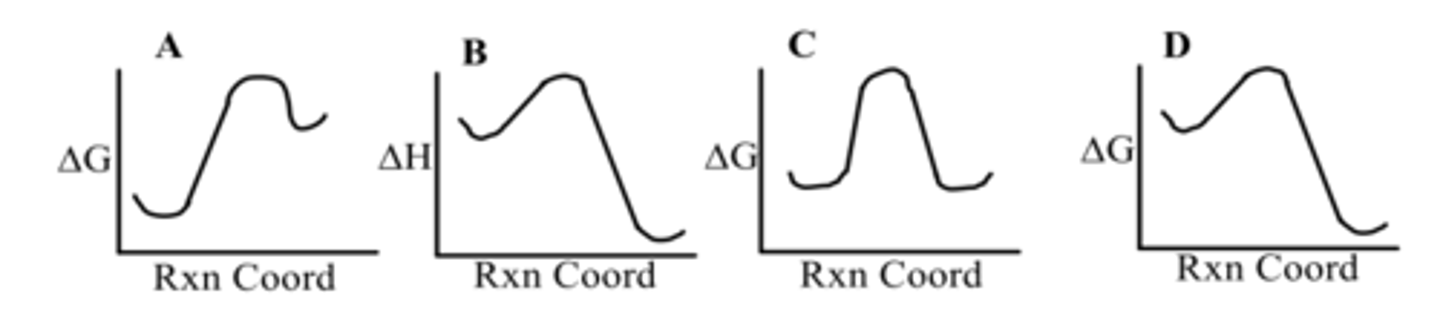
A
Which diagram shows an endergonic reaction?
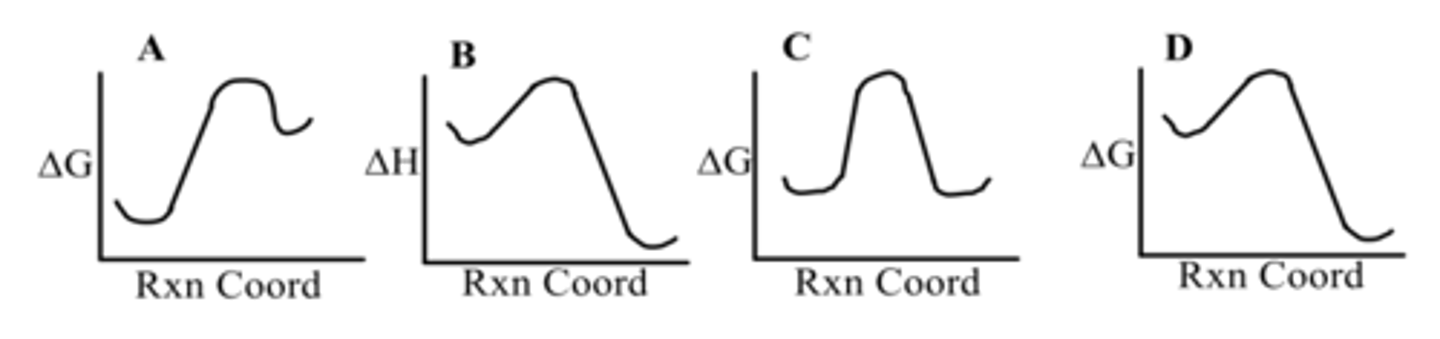
B
Which diagram shows an exothermic reaction?
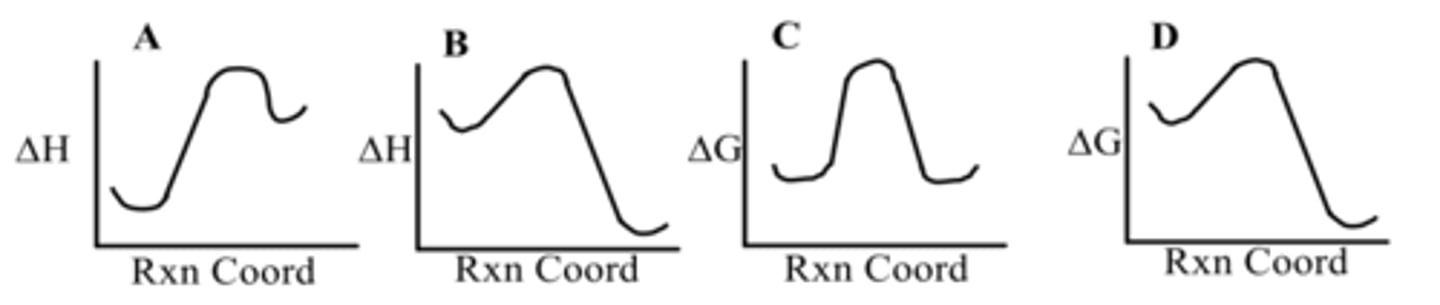
A
Which diagram shows an endothermic reaction?
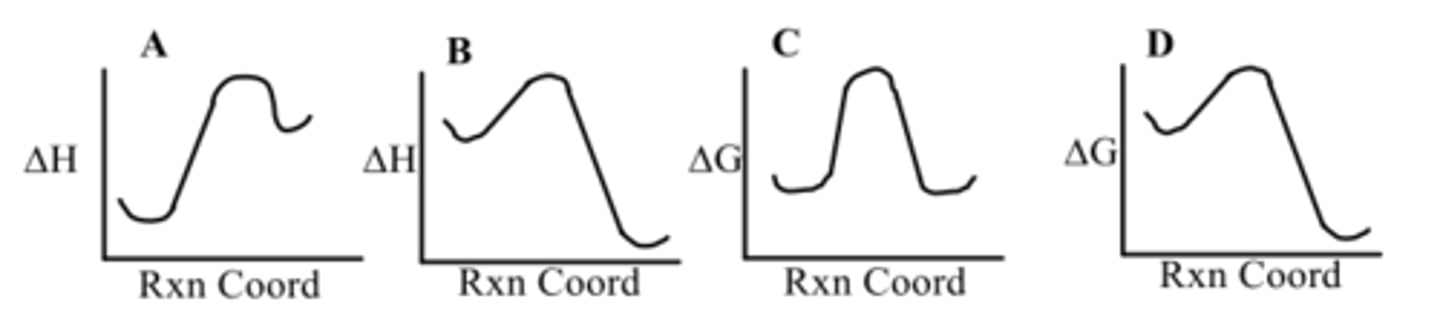
B
Which statement is consistent with the 2nd law of thermodynamics?

D
What is the first law of thermodynamics?

D
Which outcome breaks the second law of thermodynamics?

C
Which is true for a spontaneous process in a system?
(a) heat is consumed (b) heat is released to the surrounding (c) heat can be consumed or released (d) heat stays constant
B
Which is true for a spontaneous process in a system
(a) the system becomes more disordered
(b) the system could become more or less disordered
(c) the system becomes more ordered
(d) disorder of the system remains constant
B
Which is true for a spontaneous process in a system
(a) the surrounding becomes more disordered
(b) the surrounding could become more or less disordered
(c) the surrounding becomes more ordered
(d) disorder of the surrounding remains constant
A
When change happens, the entropy of the universe must
(a) increase
(b) decrease
(c) increase or decrease
(d) stay constant
C
When change happens, the entropy of the surrounding must
(a) increase
(b) decrease
(c) increase or decrease
(d) stay constant
A
Do proteins have high heat capacities / why?
a. yes, they have many possible conformations
b. no, they have very stable structures
c. no, they have many conformations
d. yes, they have very stable structures.
E
Entropy is a measure of
a) probability
b) disorder
c) heat distribution
d) answers (a) and (b)
e) all the above
C
All processes must be favorable under
a specific condition if they are a. exothermic
b. endothermic
c. exergonic
d. endergonic
e. more free
B
The bond between Na+ and Clis weakest in
a. hexane
b. water
c. methanol
d. acetone
A
Which diagram depicts a nonspontaneous reaction?
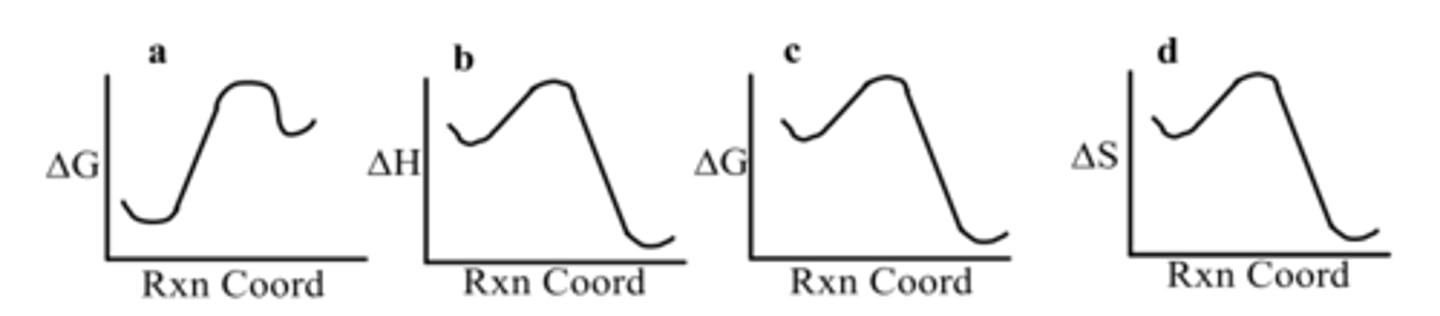
D
Which functional group can form a off-faced stack arrangement?
a. carboxylic acid
b. amides
c. alcohol
d. arenes
e. amines
B
The Gibbs free energy for a chemical process was -8 kcal/mol and the heat produced was -5 kcal/mol. What was the TS term for this process?
a. -3 kcal/mol
b. 3 kcal/mol
c. -13 kcal/mol
d. 13 kcal/mol
A
The enthalpy for a chemical process was 2 kcal/mol and TS was 5 kcal/mol. What was the Gibbs free energy (G) term for this process?
a. -3 kcal/mol
b. 3 kcal/mol
c. -7 kcal/mol
d. 7 kcal/mol
C
The Gibbs free energy for a chemical process was -5 kcal/mol and TS was -2 kcal/mol. What was the enthalpy for this process?
a. -3 kcal/mol
b. 3 kcal/mol
c. -7 kcal/mol
d. 7 kcal/mol
A
The Gibbs free energy for a chemical process was 4 kcal/mol and the heat produced was 3 kcal/mol. What was the TS term for this process?
a. -7 kcal/mol
b. 7 kcal/mol
c. -1 kcal/mol
d. 1 kcal/mol
D
The Gibbs free energy for a chemical process was -13 kcal/mol and TS was -2 kcal/mol. What was the enthalpy for this process?
a. 15 kcal/mol
b. 11 kcal/mol
c. -11 kcal/mol
d. -15 kcal/mol
B
A thermometer measures
(a) thermo energy
(b) average kinetic energy
(c) potential energy
(d) heat
D
Which reaction can be coupled to A + B = C + D G = 3 kcal/mol?

A
The bond between Na+ and Clis strongest in
a. hexane
b. water
c. methanol
d. acetone
A
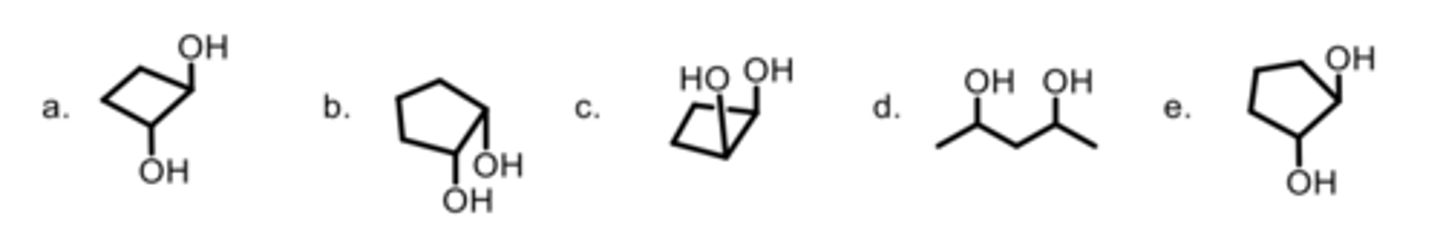
Rank the strength of the interactions between the molecules from the highest to lowest.
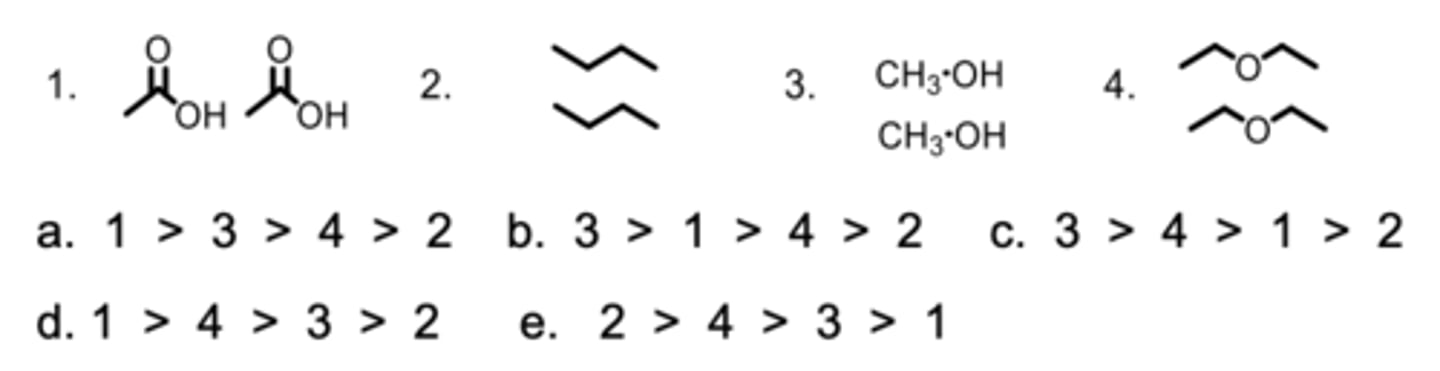
A
Which phosphate would be linked to the hydroxyl group of glucose during enzyme catalysis?
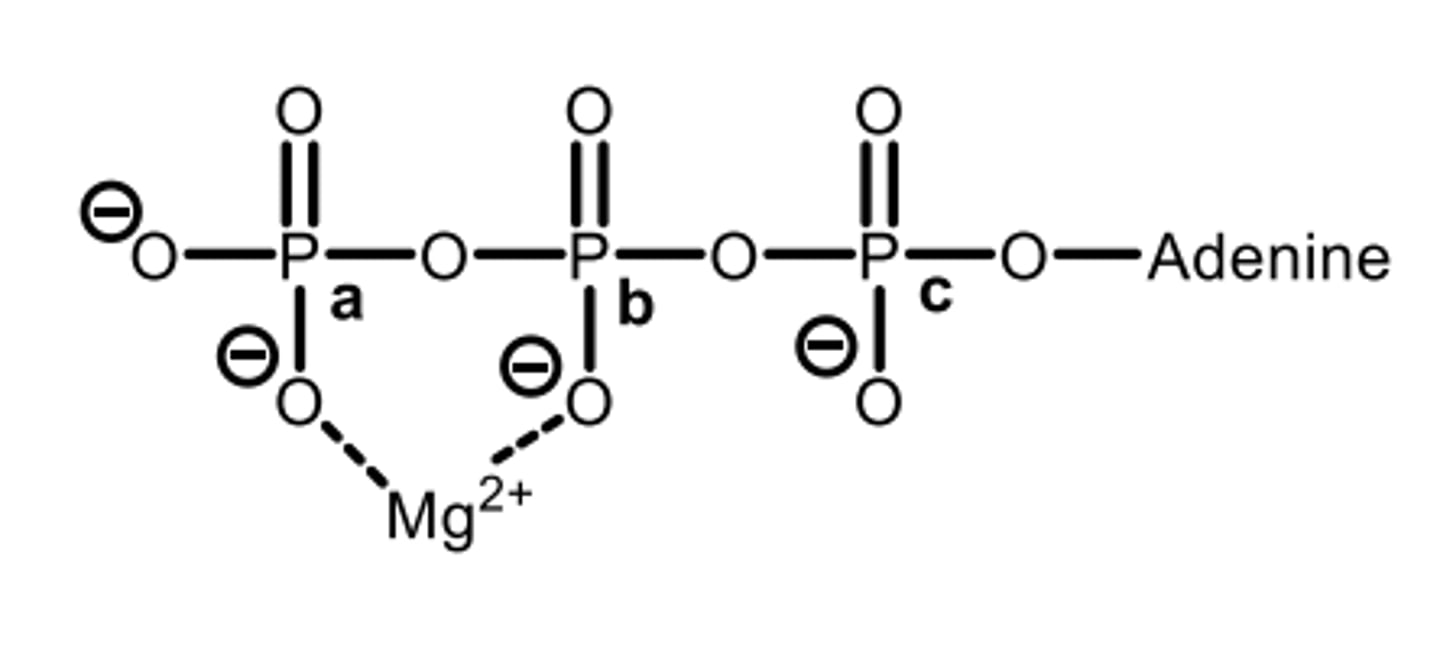
B
Rank the strength of the interactions between the molecules from the highest to lowest.
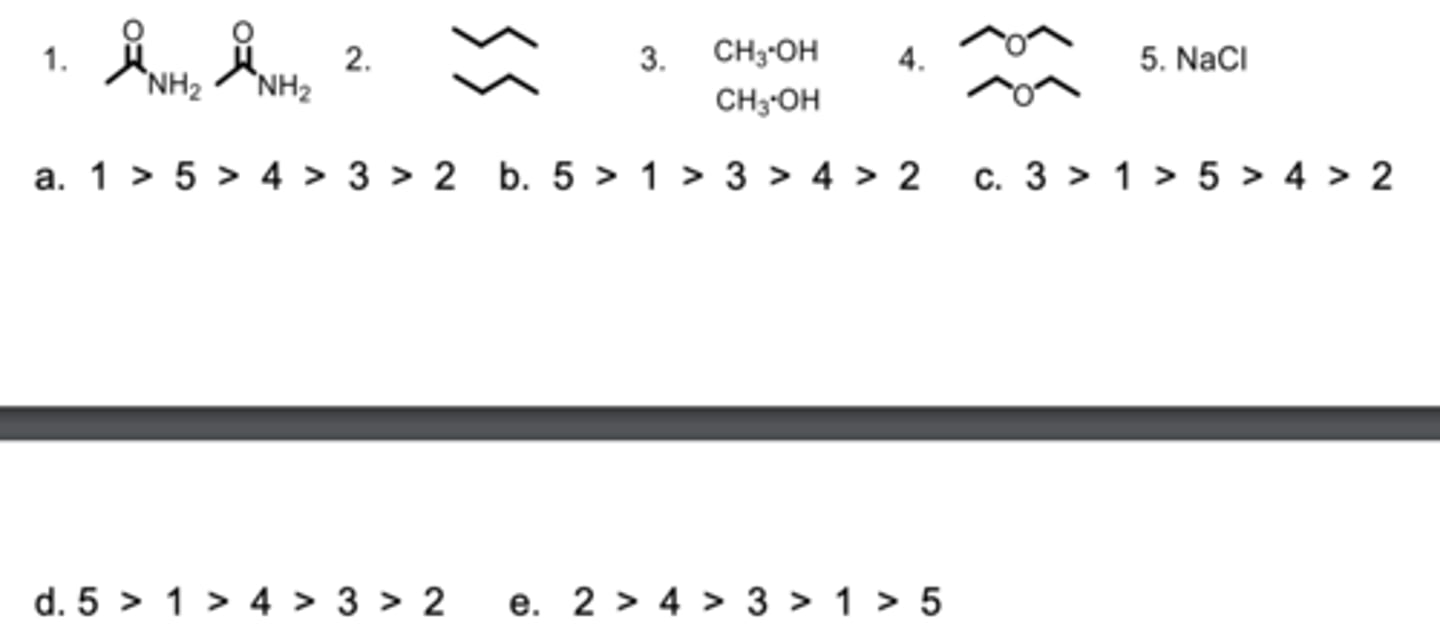
D
Which electrostatic interaction is less affected by going from the gas phase to water?
a. ionic
b. H-bond
c. dipole
d. dispersion
B
Which functional group can form a T-stack arrangement?
a. carboxylic acid
b. arene
c. alcohol
d. amides
e. amines
E
Which sign/parameter shows an exothermic process?
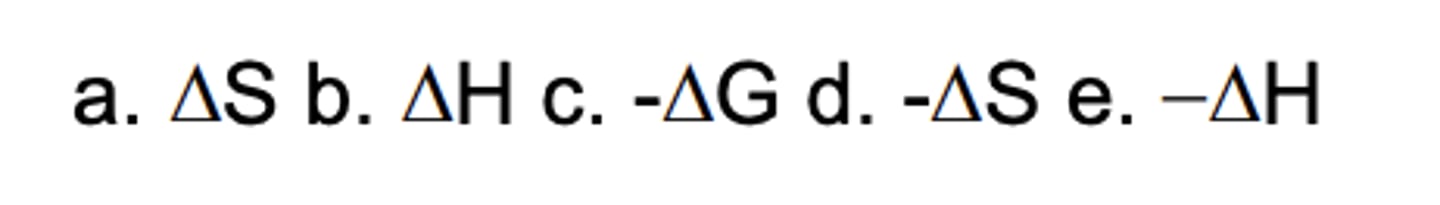
C
Which sign/parameter shows an exergonic process?
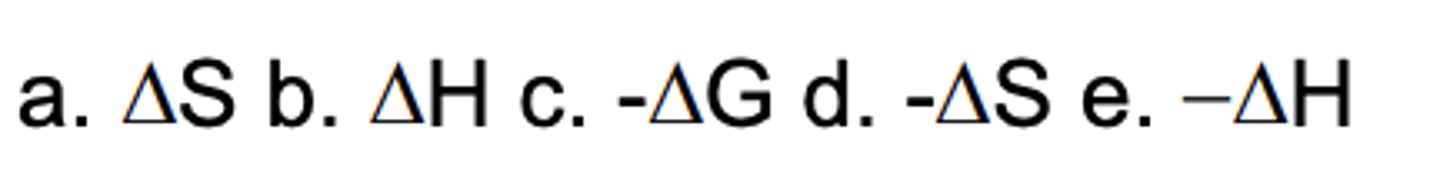
A
. Which sign/parameter shows an endergonic process?
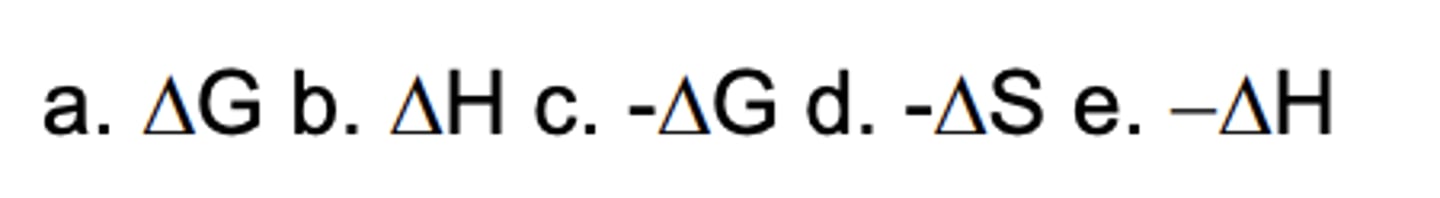
A
Which sign/parameter shows a gain in freedom during a process?
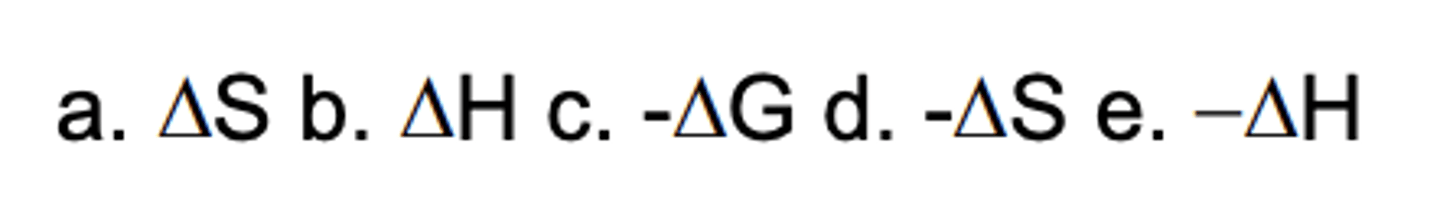
D
Which sign/parameter shows a loss of freedom during a process?
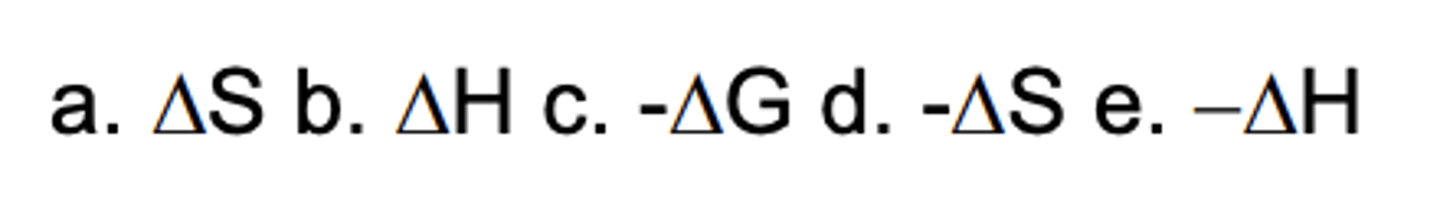
B
Which sign/parameter shows an endothermic process?
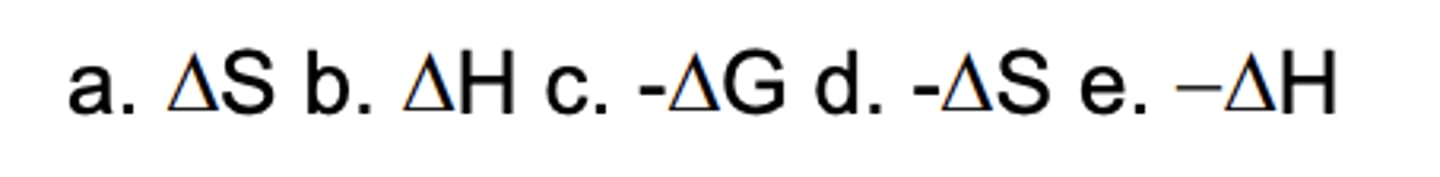
D
Which parameter gives the distribution of heat in a system?
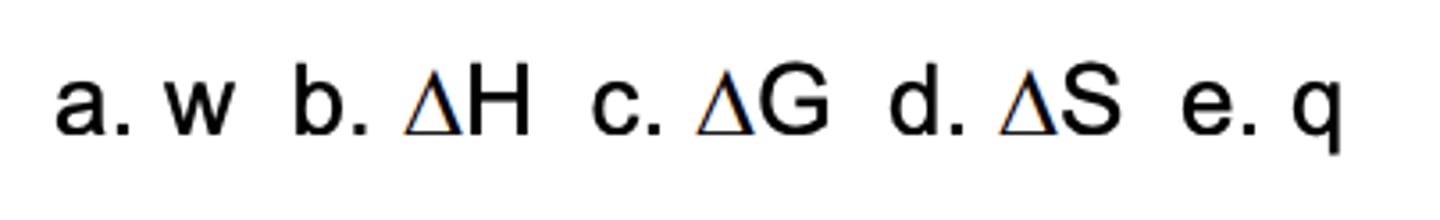
B
. Which is the first law of thermodynamics?
a. Energy can be created and destroyed
b. Energy cannot be created or destroyed
c. Energy can be destroyed and not created
d. Energy can be created and not destroyed
D
Which statement is consistent with the 2nd law of thermodynamics?
a. The entropy of the universe cannot be destroyed
b. The entropy of a system always increases
c. Cold objects will spontaneously give energy to warm objects
d. Freedom of motion of a system can decrease
C
A thermometer measures
a. pressure
b. heat
c. average kinetic energy
d. molecular conformations
D
Which is not wasted heat?
a. Conformational changes
b. Increased molecular vibrations
c. Breaking H-bonds
d. Expanding glassware
A
Which system has the highest entropy?

C
A spontaneous reaction
a. Has a positive free energy
b. Makes less stable molecules
c. Makes more stable molecules
d. Happens immediately once the materials are mixed
D
Which reaction can be coupled to A + B = C + D G = 3 kcal/mol?

C
Which reaction can be coupled to A + B = C + D G = 3 kcal/mol?
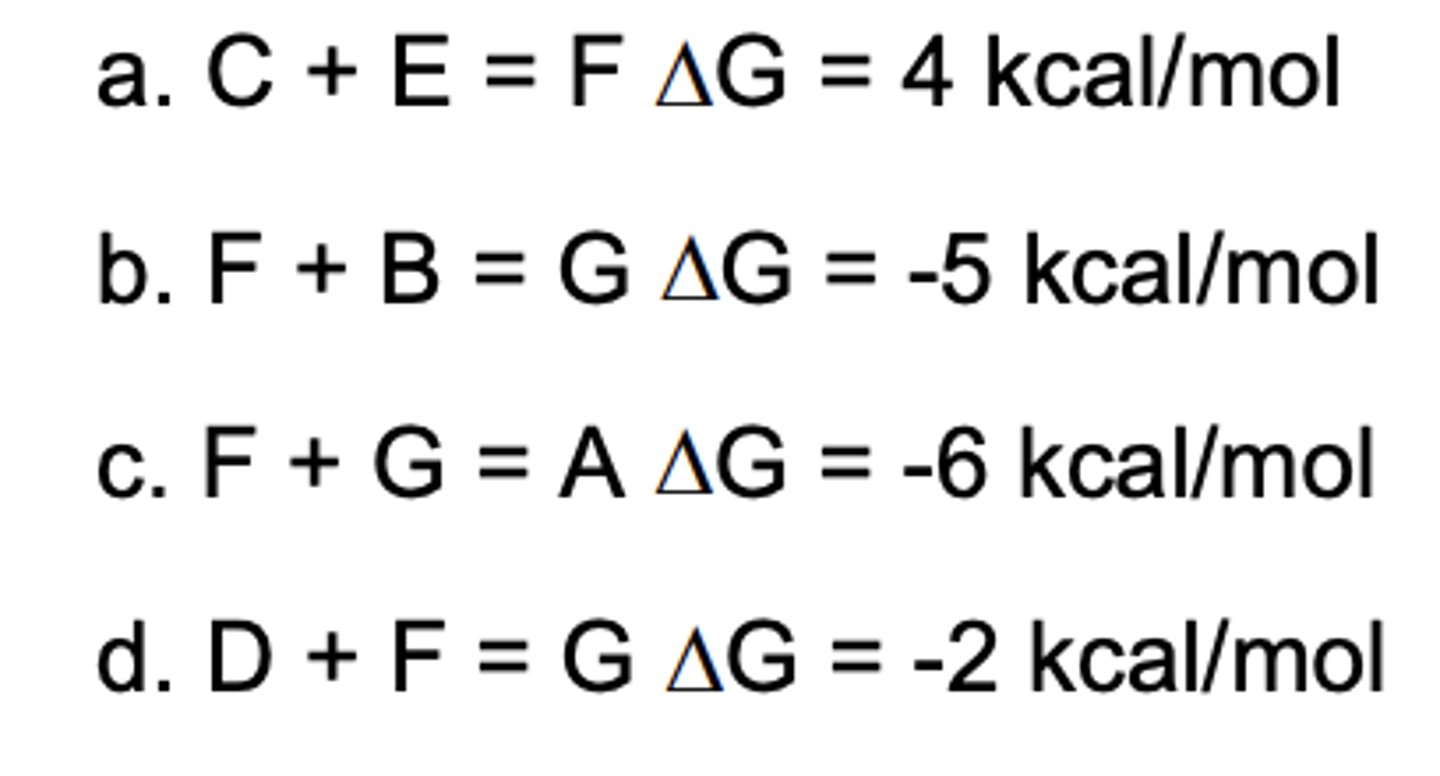
E
Which are symbols for heat
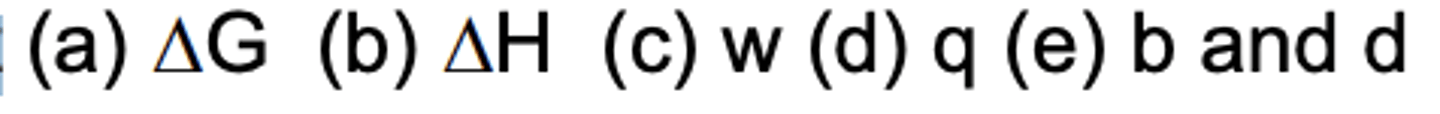
D
Entropy is a measure of
(a) disorder
(b) probability
(c) energy distribution
(d) all answers
C
A chemical process gives off heat to the surrounding and the system becomes more ordered. The process is
a. spontaneous
b. nonspontaneous
c. its spontaneity cannot be determined
B
A chemical process takes heat from the surrounding and the system becomes more ordered. The process is
a. spontaneous
b. nonspontaneous
c. its spontaneity cannot be determined
A
A chemical process gives off heat to the surrounding and the system becomes more disordered. The process is
a. spontaneous
b. nonspontaneous
c. its spontaneity cannot be determined
D
The Gibbs free energy for a chemical process was -5 kcal/mol and the heat produced was -7 kcal/mol. What was the TS term for this process?
a. -12 kcal/mol
b. 12 kcal/mol
c. 2 kcal/mol
d. -2 kcal/mol
B
Which reaction can be coupled to A + B = C + D G = 3 kcal/mol?

B
Which phosphate would be linked to the hydroxyl group of a sugar?
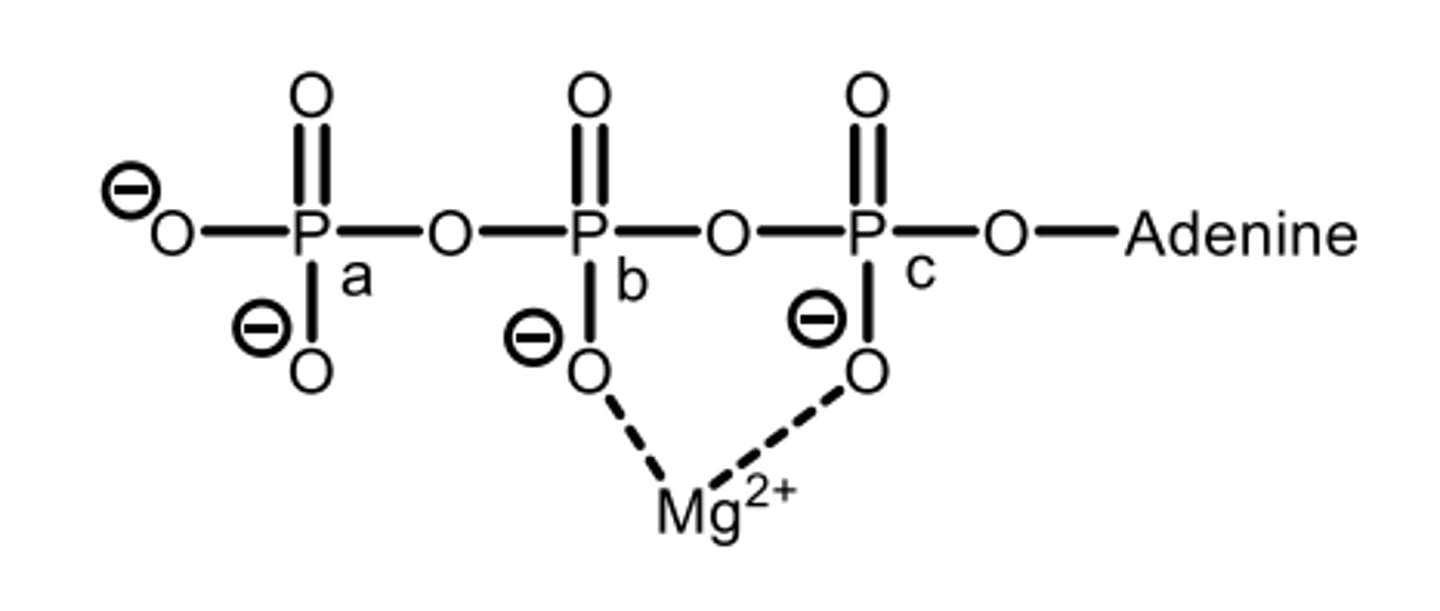
D
Fe3+ is called a _____ when bound to a protein and is chemically active.
a. substrate
b. coenzyme
c. cosubstrate
d. cofactor
A
. A organic molecule that is loosely bound by a protein is called a
a. cosubstrate
b. prosthetic group
c. substrate
d. particle
B
A organic molecule that is tightly bound by a protein is called a
a. cosubstrate
b. prosthetic group
c. substrate
d. particle
A
Which vitamin is responsible for eye health?
a. A
b. B
c. C
d. D
e. E
f. K
C
Which vitamin is used to make collagen?
a. A
b. B
c. C
d. D
e. E
f. K
B,C
Which vitamins are water soluble?
a. A
b. B
c. C
d. D
e. E
f. K
F
Which vitamin is responsible for blood clotting?
a. A
b. B
c. C
d. D
e. E
f. K
d
Which vitamin is responsible for bone health?
a. A
b. B
c. C
d. D
e. E
f. K