Biological molecules - Biology A-Level OCR A
1/133
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
134 Terms
What is a condensation reaction?
A reaction that occurs when two molecules are joined together with the removal of water
What is a hydrogen bond?
A weak interaction that can occur wherever molecules contain a slightly negatively charged atom bonded to a slightly positively charged hydrogen atom
What is a hydrolysis reaction?
A reaction that occurs when a molecule is split into two smaller molecules with the addition of water
What is a monomer?
A small molecule which binds to many other identical molecules to form a polymer
What is a polymer?
A large molecule made from many smaller molecules called monomers
What is a covalent bond?
A chemical bond formed when two or more atoms share electrons.
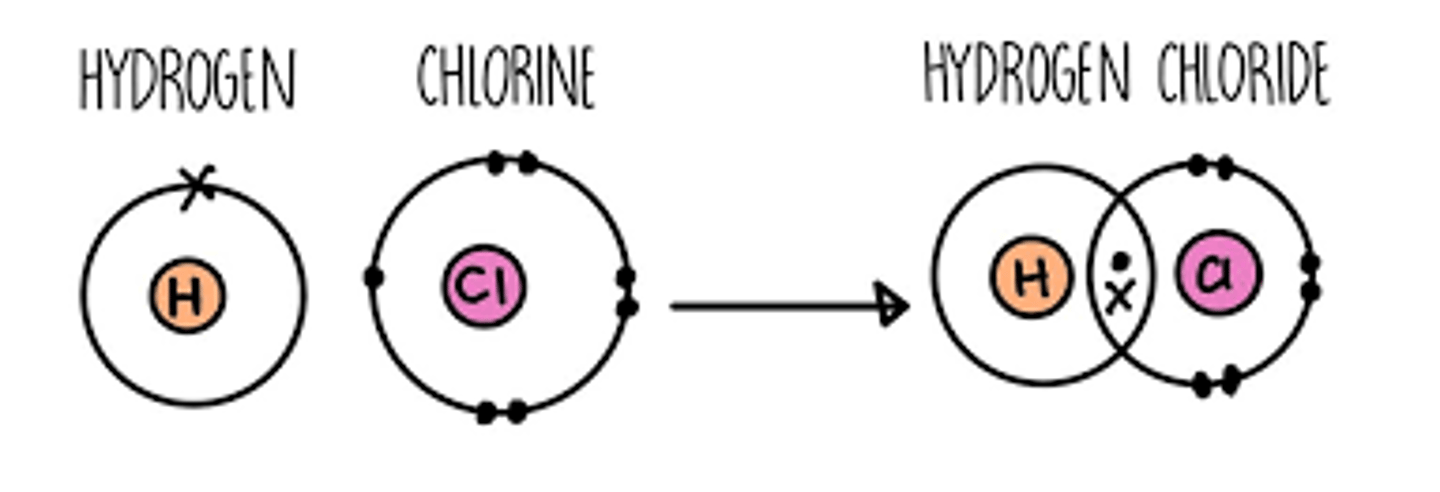
What is it called when two molecules are joined together?
A dimer
What is the monomer and the polymer of Carbohydrates?
Monomer - Monosaccharides (e.g. glucose)
Polymer - Polysaccharides (e.g. starch)
What is the monomer and the polymer of Proteins?
Monomer - Amino acids
Polymer - Polypeptides
What is the monomer and the polymer of Nucleic acids?
Monomer - Nucleotides
Polymer - DNA/RNA
What does it mean for molecules to be polar?
Oxygen atom becomes slightly negative and the hydrogen atoms become slightly positive
What are the properties of water?
Liquid, Density, Solvent, Cohesion and surface tension, High specific heat capacity, High latent heat of vaporisation, Reactant
Liquid
- Water molecules constantly move around. Unlike other liquids, they continually make and break hydrogen bonds
Because water is liquid at room temperature, it can...
- Provide habitats for living things in rivers, lakes and seas
- Form a major component of tissues in living organisms
- Provide a reaction medium for chemical reactions
- Provide and effective transport medium
Density
- When most liquids get colder, they become more dense. If this were the case with water, the top of a pond would freeze and sinks nether water replacing it at the top would continue to do the same until the whole pond is frozen
- Water behaves differently. It gets more dense as it becomes colder until about 4 degrees Celsius. As it goes from 4 degrees to freezing point, the molecules align themselves in a structure that is less dense than liquid water due to their polar nature
Because ice is less dense than water...
- Aquatic organisms have a stable environment to live in through the winter
- Ponds and other bodies of water are insulated against extreme cold as the layer of ice reduces the rate of heat loss from the rest of the pond
Solvent
- water is a good solvent for many substances founding living things.
- Because water is polar, the positive and negative parts of the water molecules are attracted to the positive and negative parts of the solute.
- The water molecules cluster around these charged parts of the solute molecules or ions and will help separate them and keep them apart.
- At this point they dissolve and a solution is formed
Because water is such a good solvent:
- Molecules and ions can move around and react together in water. Many reactions happen in the cytoplasm of cells, which is 70% water.
- Molecules and ions can be transported around living things whilst dissolved in water
Cohesion and surface tension
- A drop of water on a flat surface does not spread out. Hydrogen bonding between molecules pulls them together in an almost-spherical shape
- The water molecules demonstrate cohesion
- This happens at the surface of water as well. The water molecules at the surface are all hydrogen bonded ro the molecules beneath them, hence they are more attracted to the water molecules beneath them than the air above
- This means the surface of the water contracts and it gives the surface of the water the ability to resit force applied to it. This is known as surface tension
Because of cohesion and surface tension:
- Columns of water in plant vascular tissue are pulled up the xylem tissue together from the roots
- Insects like pond skaters can walk on water
High specific heat capacity
Water molecules are held together quite tightly by hydrogen bonds, so in order to increase their kinetic energy and temperature, a lot of heat energy is required.
This means that water does not heat up or cool down quickly
What is specific heat capacity?
The amount fo heat energy required to raise the temperature of 1kg of water by 1 degrees celcius
Why high specific heat capacity of water is important:
- Living things need a stable temperature for enzyme-controlled reactions to happen properly
- Aquatic organisms need a stable environment in which to live
High latent heat of vaporisation
- When water evaporates, heat energy helps the molecules break down. Because the molecules are held together by hydrogen bonds, a relatively large amount of energy is needed for water molecules to evaporate.
- Therefore, water can help to cool living things and keep their temperature stable
(e.g. mammals are cooled when sweat evaporates, and plants are cooled when water evaporates form mesophyll cells)
Reactant
Its role as a reactant is important for digestion and synthesis o large biological molecules
What are carbohydrates?
a group of molecule containing C, H and O
What is a glycosidic bond?
A bond formed between two monosaccharides by a condensation reaction
Monosaccharides
Simplest carbohydrates. They are particularly important in living things as a source of energy
How are monosaccharides suited to their role as a source of energy?
They are suited to this role because of their large number of carbon-hydrogen bonds. They are sugars, are soluble in water and are insoluble in non-polar solvents.
In what forms can monosaccharides exist?
Monosaccharides can exist as straight chains or in ring/cyclic forms.
Different sugars have different numbers of (...)
Carbon atoms.
(e.g. hexose sugars - 6 carbon atoms
pentose sugars - 5 carbon atoms
triose sugars - 3 carbon atoms)
What is an isomer?
Molecules with the same molecular formula but different structures
What is an example of isomers?
a-glucose and b-glucose
What is a disaccharide?
two monosaccharides joined together by a glycosidic bond
What is a reducing sugar?
A sugar that can donate electrons to (or reduce) another chemical.
Features of disaccharides
Sweet and soluble
Which two monosaccharides join to make maltose?
a-glucose + a-glucose
Which two monosaccharides join to make sucrose?
a-glucose + fructose
Which two monosaccharides join to make lactose?
b-galactose + a-glucose
Which two monosaccharides join to make cellobiose?
b-glucose + b-glucose
What is the molecular formula for a-glucose?
C6H12O6
What is the molecular formula of b-glucose?
C6H12O6
What is the molecular formula for ribose?
C5H10O5
What is the molecular formula for deoxyribose?
C5H10O4
What is the role of a-glucose in the body?
- Energy source
- Component of starch and glycogen, which act as energy stores
What is the role of b-glucose in the body?
- Energy source
- Component of cellulose, which provides structural support in plant walls
What is the role of ribose sugars in the body?
Component of RNA, ATP and NAD
What is the role of deoxyribose sugars in the body?
Component of DNA
What type of sugars are b-glucose and a-glucose?
They are hexose sugars
What type of sugars are deoxyribose and ribose sugars?
They are pentose sugars
What are polysaccharides?
Polymers of monosaccharides
What are homopolysaccharides?
Polysaccharides made of solely one type of monosaccharide
What are heteropolysaccharides?
Polysaccharides made of more than one types of monomer
Respiration formula
glucose + oxygen --> carbon dioxide + water
C6H12O6 + 6O2 -> 6CO2 + 6H2O
Why polysaccharides are good energy stores?
- Glycogen and starch are compact - dont occupy much space
- Polysaccharides hold glucose molecules in chains - the glucose molecules can be easily 'snipped off' from the chain by hydrolysis when required for respiration
- Some chains are unbranched and some are branched.
- polysaccharides are less soluble in water than monosaccharides - do not affect the water potential
Branched chains of glucose
- Tend to be more compact than unbranched chains
- Offer the chance for lots of glucose molecules to be snipped off by hydrolysis at the same time when lots of energy is required quickly.
What enzyme is responsible for hydrolysing 1-4 linkages?
Amylase
What enzyme is responsible for hydrolysing 1-6 linkages?
Glucosidase
Amylose
- Found in plants
- Long chain of a-glucose molecules
- 1-4 glycosidic bonds
Structure of amylose
- Coils into a spiral shape, with hydrogen bonds holding the spiral in place
- Hydroxyl groups on carbon 2 are situated inside teh coil, making the molecule less soluble and allowing hydrogen bonds to form to maintain the coils structure
Amylopectin
- Found in plants
- Has glycosidic bonds between carbon 1-4
- In addition, it has branches formed by glycosidic bonds between carbons 1-6
Structure of amylopectin
- Coils into a spiral shape, like amylose, and is held together by hydrogen bonds but with branches emerging from the spiral
Glycogen
- Found in animals
- Glycosidic bonds between carbon 1-4 and branches formed by glycosidic bonds between carbon 1-6
Structure of glycogen
- The 1-4 carbon chains tend to be smaller than in amylopectin
- Thsi means it has less tendency to coil
- It has more branches, making it more compact
- Easier to remove more monomer units as there are more ends
Cellulose as a structural unit
- Found in plants and forms the cell walls
- It is tough, insoluble and fibrous
- Cellulose is a homopolysaccharide made of long chains of up to 15,000 b-glucose molecules, bonded together through condensation reactions to form glycosidic bonds
How is the difference in structure in cellulose a direct cause of bonding?
- Hydrogen and hydroxyl groups on carbon 1 are inverted in b-glucose compared to a-glucose. This means that every other b-glucose molecule in the chain is inverted. This and the b-1-4 glycosidic bond help to prevent the chain spiralling
- Hydrogen bonding between the roatated b-glucose molecules in each chain also gives the chain additional strength and stops it spiralling
- Hydrogen bonding between the rotated b-glucose molecules in different chains gives the whole structure additional strength. the hydroxyl group on carbon 2 sticks out, enabling hydrogen bonds to be formed between chains
What are microfibrils?
Neighbouring chains of glucose molecules (60 to 70 chains) linked by hydrogen bonds.
What are macrofibrils?
Up to 400 microfibrils bundled togetehr which are embedded in pectins to form plant cell walls
Structure of cell walls
- Microfibrils and macrofibrils both have very high tensile strength because of the glycosidic bonds and the hydrogen bonds between chains
- Macrofibrils run in all directions, criss-crossing the wall for extra strength
- It is difficult to digest cellulose because the glycosidic bonds between the glucose molecules are less easy to break. Most animals do not have the enzyme to catalyse the reaction
How the structure of the cell wall helps it do its jobs?
- Because plants dont have a rigid skeleton, each cell needs to have enough strength to support the plant
- There is a space between macrofibrils for water and mineral ions to pass on their way into and out of the cell. This makes the cell wall fully permeable
- The wall has high tensile strength, which prevents the plant cells from bursting when they are turgid, which supports the whole plant
- The macrofibril structure can be reinforced with other substances for extra support or to make the walls water proof
What are two other structural polypeptides?
Bacterial cell walls - the whole structure surrounding the cell is called peptidoglycan, made from long polypeptide chains that lie in parallel, cross-linked by short peptide chains made of amino acids
Exoskeletons - Insect and crustacean exoskeletons are made of chitin.
How cellulose differs from chitin?
Chitin has an acetylamino group rather than a hydroxyl group on carbon 2. It forms cross-links between long parallel chains of acetyglucosamine in a similar way to cellulose
What are lipids?
A group of substances that are soluble in alcohol rather than water
Examples of lipids include
Triglycerides, phospholipids, glycolipids and cholestrol
What is a macromolecule?
A very large, organic molecule
What is a phospholipid?
A molecule consisting of glycerol, two fatty acids and one phosphate group
What is the structure of triglycerides?
Triglycerides-have 3 fatty acids and 1 glycerol. Can be saturated or unsaturated.
Glycerol
a three-carbon alcohol with a hydroxyl (-OH) groupS attached to each carbon
Fatty acids
fatty acids have a carboxyl group (-COOH) on one end attached to a hydrocarbon tail made of only carbon and hydrogen. This can be anything from 2-20 carbons long
What does it mean for a fatty acid to be saturated?
It means that there are no C=C bonds in the molecule
What does it mean for a fatty acid to be unsaturated?
There is a double bond between two carbon atoms, meaning fewer hydrogen atoms can be bonded to the molecule
What does it mean for a fatty acid to be monounsaturated?
There is only one C=C bond.
What does it mean for a fatty acid to be polyunsaturated?
There is more than just one C=C bond
How does having one or more C=C bond change the shape of the hydrocarbon chain?
It gives the hydrocarbon chain a kink where the double bond is. Because these kinks push the molecules apart slightly, it makes them more fluid
What are ester bonds?
An ester bond is formed when a molecule having the carboxylic group reacts with another molecule having a hydroxyl group. The carboxylic group loses its hydrogen and oxygen while the alcohol loses hydrogen of its hydroxyl group.
Functions of triglycerides
Energy source
Energy store
Insulation
Buoyancy
Protection
Triglycerides an an energy source
Triglycerides can be broken down in respiration to release energy and generate ATP.
The first step is to hydrolyse the ester bonds, and then both glycerol and fatty acids can be broken down completely to CO2 and water.
Which produces more water, respiration of a lipid or of a sugar?
The respiration of a lipid produces more water than the respiration of a sugar
Triglycerides as an energy store
Because triglycerides are insoluble in water, they can be stored without affecting the water potential of the cell
Triglycerides as insulation
Adipose tissue is a storage location for lipids in whales, acting as a heat insulator. Lipid in nerve cells acts as an electrical insulator. Animals preparing for hibernation store extra fat
Triglycerides and buoyancy
Because fat is less dense in water, it is used by aquatic animals to help them stay afloat
Protection
Humans have fat around delicate organs, such as their kidneys, to act as a shock absorber. the petidoglycan cell wall of some bacteria is covered in a lipid rich outer coat
What is the structure of phospholipids?
Phospholipids have the same structure as triglycerides, except one of the fatty acids it replaced by a phosphate group
Behaviour of phospholipids in water
- When surrounded by water, he phosphate group has a negative charge, making it polar
- The fatty acid has a positive charge, making it non-polar and so the tails are repelled by water.
- The head is referred to as hydrophilic while the tail is referred to as hydrophobic
- This makes the phospholipid molecule amphipathic
Distinct properties of amphipathic phospholipids
- They form a layer on the surface of water with heads in the water and tails sticking out
- They may form micelles
What is a micelle?
Tiny balls with the tails tucked away inside and the heads pointing outwards towards the water
Phospholipid bilayer
Two rows of phospholipids with the tails point inwards and the heads sticking outwards in the solution. Between 20-80% of membranes in plant and animal cells are made of phospholipids.
- The individual phospholipids are free to move around in their layer, but will not move into any position in which their hydrophobic tails will be exposed to water, which gives the membrane some stability
- The membrane is selectively permeable. it is only possible for small and non-polar molecules to move through the tails in the bilayer, such as oxygen and carbon dioxide. This lets the membrane control what goes in and out of the cell, and keeps it functioning properly.
Cholesterol
- Steroid alcohol (sterol)
- Small and hydrophobic, so can sit in the middle of the bilayer
- It is mainly made in the liver of animals and plants also have a cholesterol derivative in their membranes. (It is called stigmasterol and is different from cholesterol in one aspect: it has a double bond between carbon 22 and 23)
What is a sterol alcohol?
A type of lipid that is not made from glycerol or fatty acids. It consists of four isoprene units (carbon based rings)
What is the role of cholesterol in the membrane?
It regulates the fluidity of the membrane, preventing it from becoming too fluid or too stiff.