Tertiary Structure
1/27
Earn XP
Description and Tags
…and how a knowledge of biochemistry could allow you to save all of humanity
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
Would “mirror image” proteins made of D-amino acids function?
microscopic ‘mirror life’ could wipe out humanity - existential threat
D-enzyme protease?
How can we stop mirror microbes?
peptoids disrupt the membranes of a vast range of “normal” microbes - achiral!
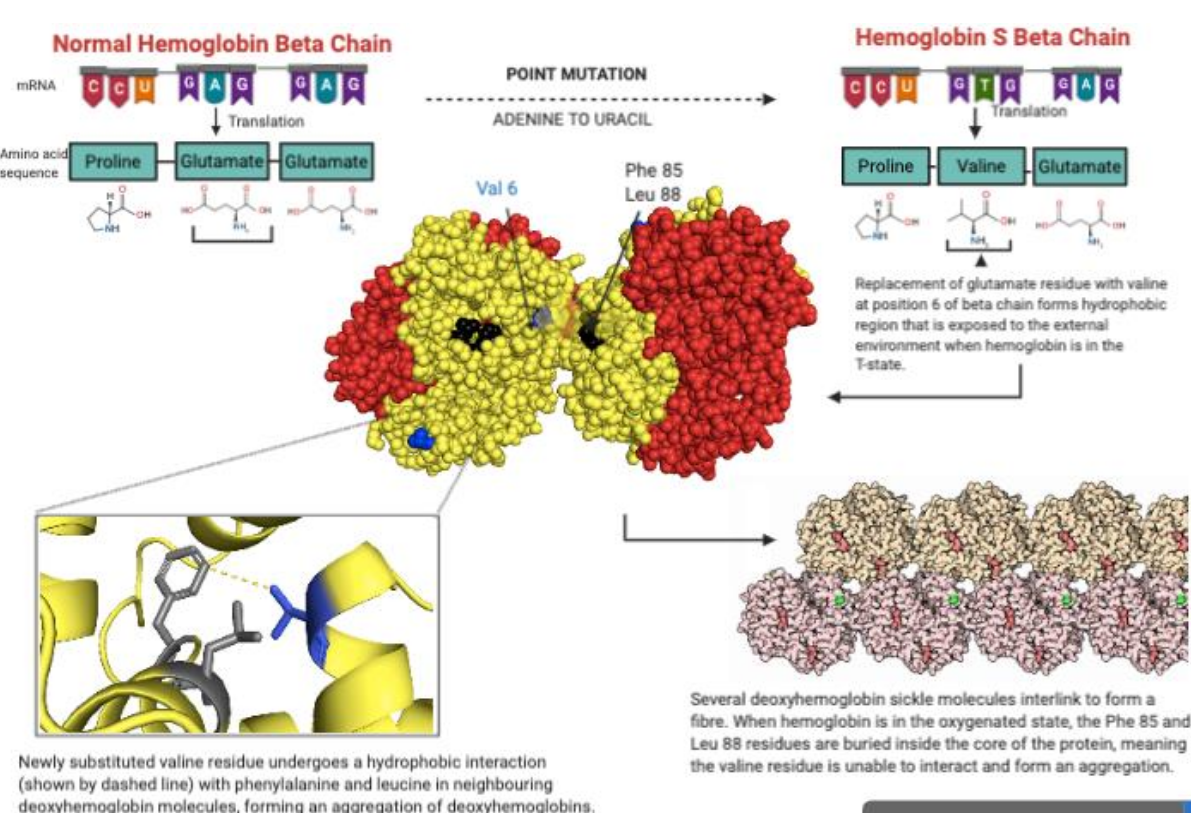
Molecular Basis of Sickle Cell Anemia
point mutation of adenosine to uracil in haemoglobin beta subunit gene → replaces glutamate with valine → changes structure and function of haemoglobin
Victoria Gray - CRISPR therapy
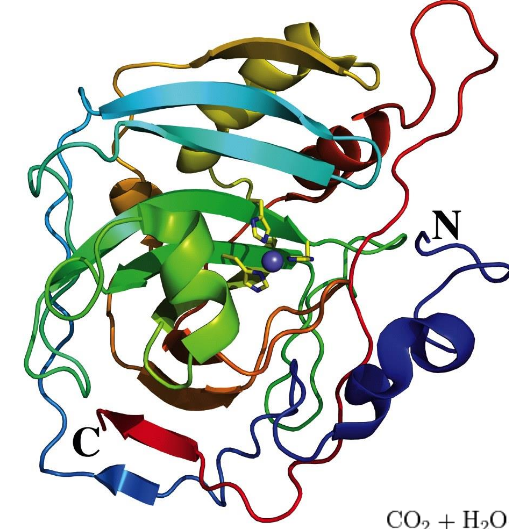
tertiary structure
3D arrangement of all the parts in a protein; an assembly of distinct secondary structures
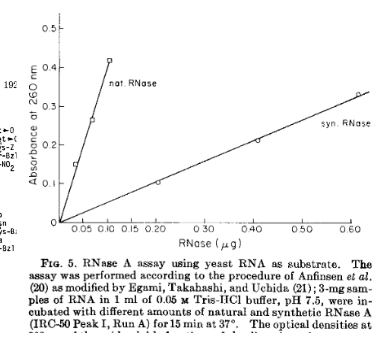
If we synthesized a peptide sequence, would it fold on its own, or does it need direction from some cellular machinery?
sort of! not as well as natural enzymes, but it gets the job done (with time)
Studying Protein Structures
determined by X-ray crystallography, multidimensional NMR spectroscopy or cryo-electron microscopy
average protein structure
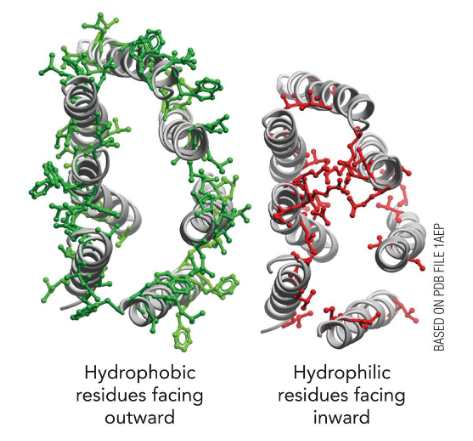
consist of one or more “domains” that are often globular in shape; Polar and charged side chains tend to lie on the outside, hydrophobic ones inside
Protein folding programs
won Nobel Prize in Chemistry 2024; studied folding of protein database and now used to design new proteins
Do individual amino acids favor a particular secondary structure type over another?
Yes but… context matters
Globular Proteins
arry out a vast range of functions (Catalysis, Transport, Signaling); Fold into compact tertiary structures; Can be modified to include additional chemical features or “prosthetic groups”
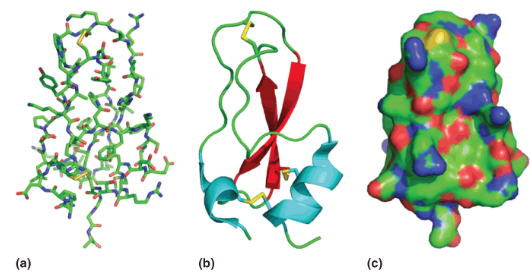
Representations of Protein Tertiary Structure
a) A “stick” model showing positions of all atoms from X-ray diffraction studies.
b) A “cartoon” model showing helices (blue), strands (red) and loops/turns (green). Disulfides also shown (yellow).
c) A ”surface” model showing the solvent accessible surface (positive in blue, negative in red, neutral in green
Protein Domains
A part of the polypeptide that is independently stable (folded); Often have distinct functions
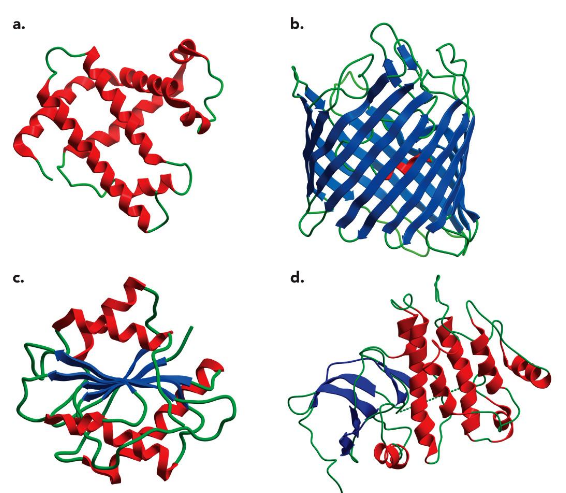
Four Major Classes of Structures
all alpha helices
all beta sheets
mixed alpha/beta
one region alpha + one region beta
Common findings in 3° Structures
Hydrophobic interactions are important. This requires two layers of secondary structure (β-α-β module for example)
Helices and sheets tend to pack in different layers because they cannot align H-bonds
Polypeptide segments close in primary sequence are usually found near each other in folded structure
Beta sheets are most stable when twisted in right-handed sense
explain the protein structure of rhodopsin?
membrane protein; folds in a non-aqueous environment
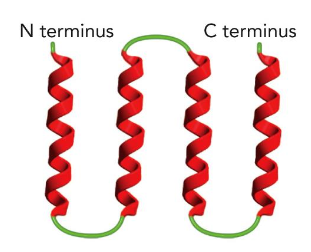
The 4-Helix Bundle
4 alpha helices connected by short loops
The Greek Key
connect beta-strands (like those coffee cup designs)
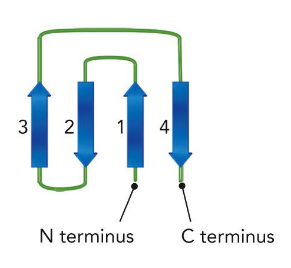
The Rossman Fold
Combining alpha-helices & beta-strands
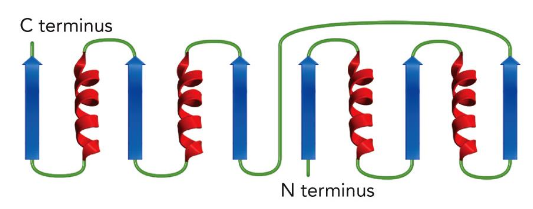
denaturation
a protein loses its functional 3-D structure
Denaturing conditions
Increased temperature
pH becomes extremely acidic or alkaline
Organic solvents or urea
How to measure denaturation
Differences between the native and denatured conformations can be detected by several spectroscopic methods; cooperativity – a steep “all-or-none” transition
measured by:
increase in solution viscosity
change in optical rotation at 365 nm
change in UV absorbance at 287 nm
Fibrous proteins
Have a filamentous, or elongated, form, usually play structural roles in animal cells and tissues
Keratins
major proteins of hair and fingernails and comprise a major fraction of animal skin; intermediate filament protein; Predominantly α-helical in structure.
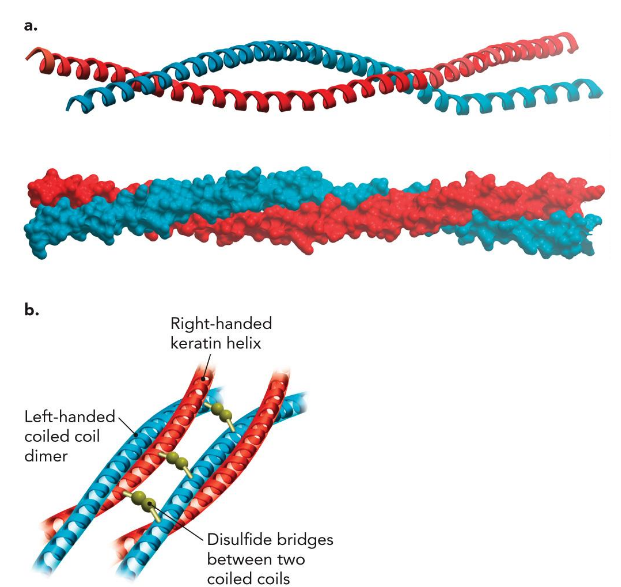
intermediate filament proteins
play important structural roles in the nuclei, cytoplasm, and surfaces of many cell types
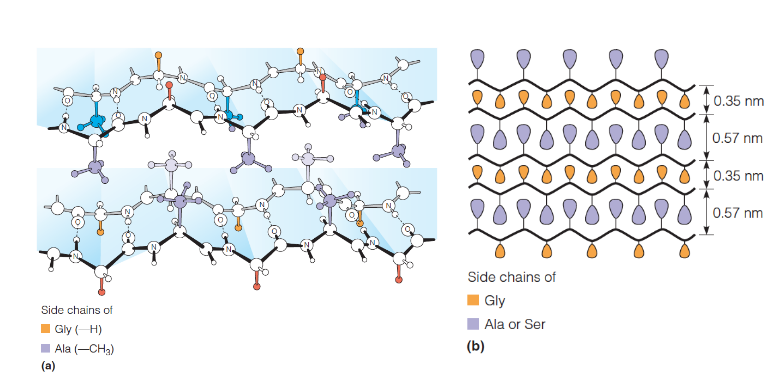
Silk Fibroin
half of its residues are glycine, other hald Ala or Ser; long regions of antiparallel β-sheet, polypeptide chains running parallel to the fiber axis
Collagen
main component of connective tissue; most abundant protein in mammals, making up about 25% to 35% of the whole-body protein content; built from triple helices of polypeptides rich in glycine and proline.
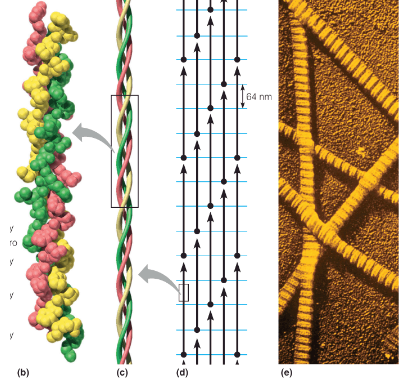
tropocollagen
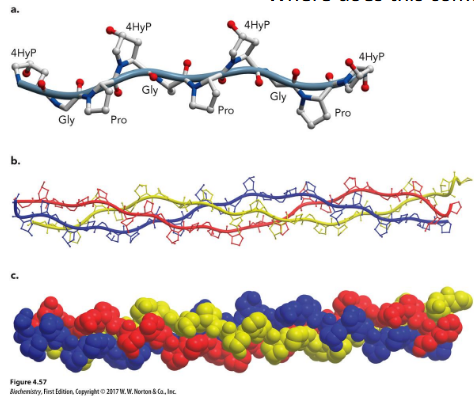
basic unit of the collagen fiber; triple helix of three polypeptide chains, ~1000 residues in length; Left-handed helices, with about 3.3 residues/turn; chains wrap around one another in a right-handed sense; Hydrogen bonds are between the chains; repetitive motif in the sequence is of the form Gly–X–Y, where X is often proline and Y is proline or hydroxyproline (4HyP; modified post-translation)
gelatin
collagen; developed in NYC by Peter Cooper