Second Law of Thermodynamics
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
major uses of second law
Identify the direction of processes
asserts that energy has quality as well as quantity
used in determining theoretical limits for the performance of commonly used engineering systems
heat engine
Devices that convert heat to work
receive heat from a high-temp source
convert part of this heat to work
reject the remaining waste heat to a low-temp sink
operate in a cycle
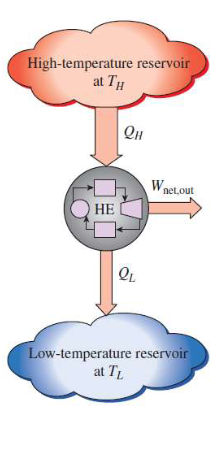
Can heat rejection be reduced in a heat engine
Every heat engine must waste some energy by transferring it to a low-temperature reservoir in order to complete the cycle, even under idealized conditions
Kelvin-Planck Statement
It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work. No heat engine can have a thermal efficiency of 100 percent, meaning that for a power plant to operate, the working fluid must exchange heat with both the environment and the furnace.
Refrigerator
The transfer of heat from a low-temperature medium to a high-temperature one requires devices called refrigerators. They most commonly operate on vapor-compression refrigeration cycles
Coefficient of Performance
The efficiency of a refrigerator or heat pump
energy efficiency rating
the amount of heat removed from the cooled space in Btu’s for 1 watthour of electricity consumed
Clasius statement
It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body. This means that a refrigerator cannot operate unless its compressor is driven by an external power source, such as an electric motor.
reversible process
a process that can be reversed without leaving any trace on the surroundings (no net heat transfer and no net work on surroundings
irreversibilities
the factors that cause a process to be irreversible
ex. friction, unrestrained expansion, mixing of two fluids, heat transfer across a finite temperature difference, electrical resistance, inelastic deformation of solids, chemical reactions
internally reversible process
if no irreversibilities occur within the boundaries of the system during the process
externally reversible
no irreversibilities within the system or its surroundings
totally reversible process
involves no irreversibilities within the system or its surroundings
involves no heat transfer through a finite temperature difference, no nonquasi-equilibirum changes, and no friction or other dissipative effects
carnot cycle
Reversible Isothermal Expansion (process 1-2, TH = constant)
Reversible Adiabatic Expansioncar (process 2-3, temperature drops from TH to TL)
Reversible Isothermal Compression (process 3-4, TL = constant)
Reversible Adiabatic Compression (process 4-1, temperature rises from TL to TH)

carnot heat engine
totally reversible cycle, therefore all the processes that comprise it can be reversed to become a carnot refrigerator
carnot principles
The efficiency of an irreversible heat engine is always less than the efficiency of a reversible one operating between the same two reservoirs.
The efficiencies of all reversible heat engines operating between the same two reservoirs are the same
temperature scale to use for heat engines
kelvin
coefficient of performance relationships
