Unit 1.4 - Biological Reactions are regulated by enzymes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
What are Enzymes?
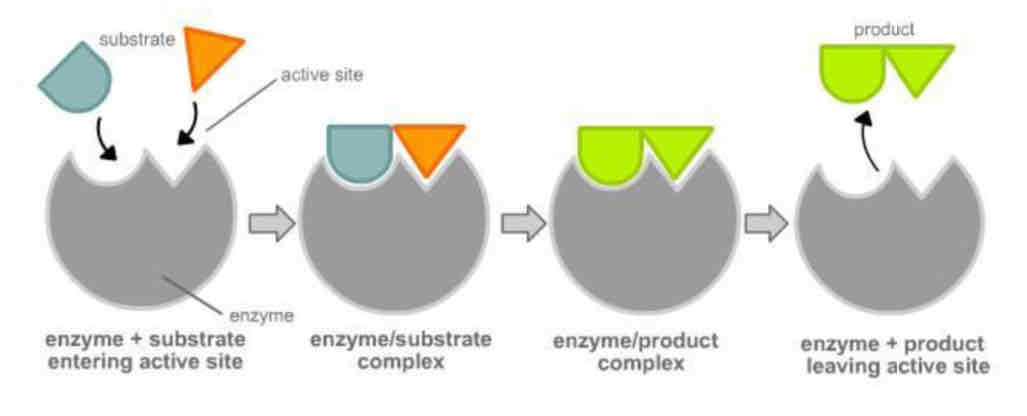
Enzymes combine with substrate molecules at the active site to produce a product.
All enzymes are tertiary proteins where the polypeptide chain is folded back on itself into a spherical globular shape.
Enzymes are biological catalysts that speed up the rate of metabolic reactions, by lowering the activation energy of the reaction. (How they do it, not definition)
What is meant by the term biological catalyst?
is a protein
Speeds up the rate of a reaction without being used themselves.
What is meant by Catalysis?
Catalysis: The lowering of the activation energy.
What is Metabolism?
Metabolism is a combination of anabolic and catabolic reactions that are catalysed by enzymes.
Enzymes - Lock and Key Hypothesis
Each enzyme reacts with particular substrate molecules – enzymes are specific.
Each enzyme has its own special 3D globular shape maintained by tertiary protein bonding.
The substrate molecule fits into and binds to an active site within the enzyme to form an enzyme-substrate complex.
The original lock and key hypothesis suggests that there is an exact fit between the substrate and the active site of the enzyme; ‘perfectly complementary’ X-ray diffraction studies of the enzyme lysozyme support this.
How does the Lock and Key Model work? - Catalysing or Hydrolising
Enzyme’s active site has a specific shape
Substrate is complementary to active site
Forms enzyme-substrate complex
Enzyme lowers activation energy needed for reaction
Products are released
An example of an anabolic reaction is shown above.
What are Anabolic & Catabolic Enzymes?
Anabolic enzymes build larger products from smaller substrate molecules.
Catabolic enzymes break large substrate molecules into smaller products.
What is Lysozyme?
Lysozyme is an enzyme found in tears and other secretions. Its function is to destroy pathogenic bacteria by breaking down their cell walls.
The bacterial cell wall is a polysaccharide consisting of chains of amino sugars. Lysozyme destroys the cell wall by breaking glycosidic bonds between the amino sugars.
X-ray diffraction has shown that there is a groove on one side of the lysozyme molecule. A section of polysaccharide, six amino sugars long, fits into the groove.
The substrate is held in place by hydrogen and ionic bonds.
The polysaccharide is broken at a specific site each time. This is a catabolic reaction.
What is the advantage of an enzyme working over a wider range of pH?
Can work in a range of areas/ pH’s around the body.
Enzymes - Induced Fit Hypothesis
Recent research suggests that the active site may not be exactly the right shape to begin with.
Scientists believe that the substrate molecule changes the shape of the active site by attaching to it ; the active site changes to fit the substrate molecule perfectly. This is called the induced fit hypothesis.
The fact that a substrate can mould the enzyme to its own shape means that several different substrates can react with the same enzyme.
This may explain the broad specificity of some enzymes e.g. lipase.
What theory represents the activity of Lysozyme?
The Induced Fit Hypothesis
Enzyme Structure
The disulphide bonds in the diagram above link different parts of the polypeptide molecule and help maintain the 3D globular shape of the enzyme, in particular the active site.
The active site is the groove in the molecule. The substrate has a complimentary shape and fits into the active site.

Enzymes - Properties
Enzymes are specific; each enzyme will catalyse only one particular reaction
Enzymes are very efficient and have a high turnover number; this means that they can convert many molecules of substrate into product per unit time.
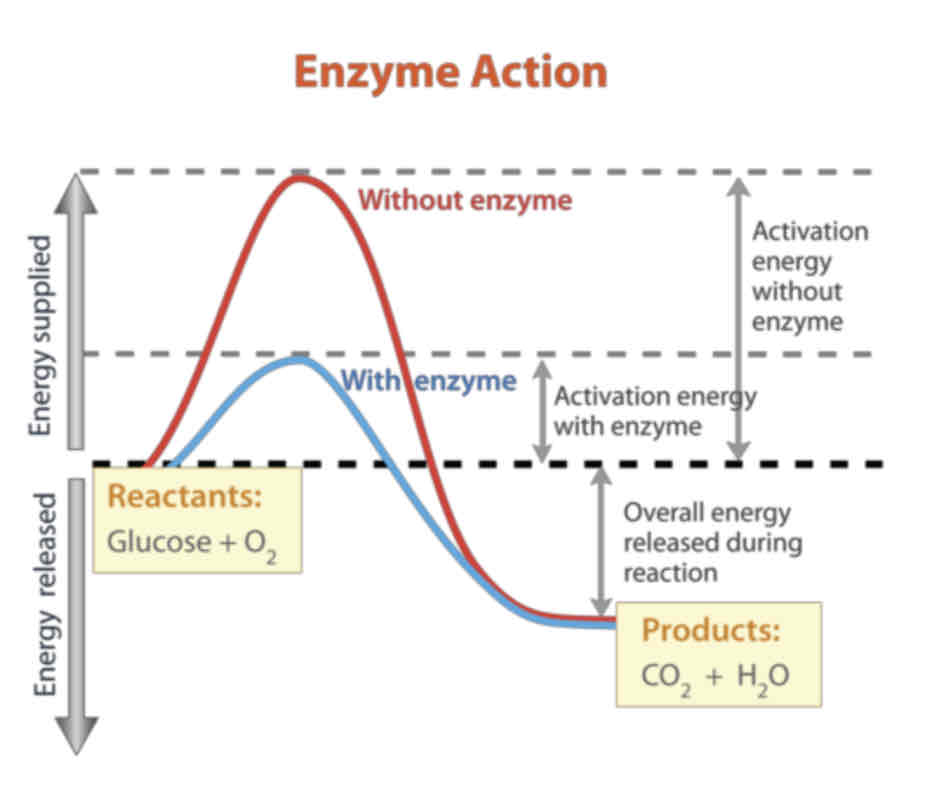
Enzymes - Activation Energy
Chemical reactions need energy to start them off; this is called activation energy. The activation energy is the energy needed to break existing chemical bonds inside molecules.
In the body, enzymes lower the activation energy of a reaction. This reduces the input of energy needed to allow reactions to take place; which means they can take place at lower temperatures.
The graph above shows the energy changes that take place during a chemical reaction.
The activation energy needed to begin the reaction is represented by the peaks. An enzyme lowers this activation energy (look at the blue curve above).
This can be exogonic/ endogonic.
What factors affect Enzyme Activity?
Changing the following factors can affect enzyme activity:
Temperature
pH
Substrate concentration
Enzyme concentration
What is the effect of Increasing Temperature? - Collision Theory
An increase in temperature gives molecules greater kinetic energy.
Enzyme and substrate molecules move around more quickly, increasing the chance of molecules colliding; this leads to the formation of more successful enzyme-substrate complexes.
Increasing the temperature of an enzyme controlled reaction results in an increase in rate of reaction (product is formed at an increased rate).
As a general rule, the rate of reaction doublesfor each 10oC rise in temperature.
This will continue until the optimum (best) temperature is reached. For most enzymes the optimum temperature is 40oC.
Enzyme Activity at 25oC
At 25 oC kinetic energy is low.
The enzyme and the substrate molecules collide less often. Fewer successful enzyme-substrate complexes form.
The product is produced slowly. Enzyme activity is low.
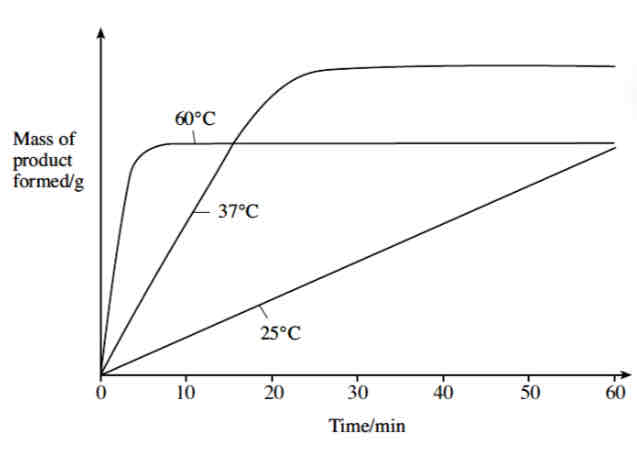
Enzyme Activity at 37oC
At 37 oC kinetic energy is higher.
The enzyme and the substrate molecules collide more often. More successful enzyme-substrate complexes form.
The product is produced more quickly (the curve is steeper between 0 and 20 minutes).
Enzyme activity levels off between 20 and 60 minutes) as substrate concentration becomes a limiting factor (substrate molecules have been converted into product).
Enzymes have different optimum temperatures - The optimum temperature of the enzyme amylase is 37oC in humans.
Human Amylase denatures at a lower temperature.
Enzyme Activity at 60oC - Excessive Heat
At 60 oC product is initially formed very quickly due to very high kinetic energy levels.
The enzymes quickly become denatured as vibrations break hydrogen bonds within the active site of the enzyme, causing the shape of the active site of the enzyme to change.
The active site is no longer complementary to the substrate.
Less product is formed as successful enzyme-substrate complex cannot form.
Unconverted substrate molecules remain.
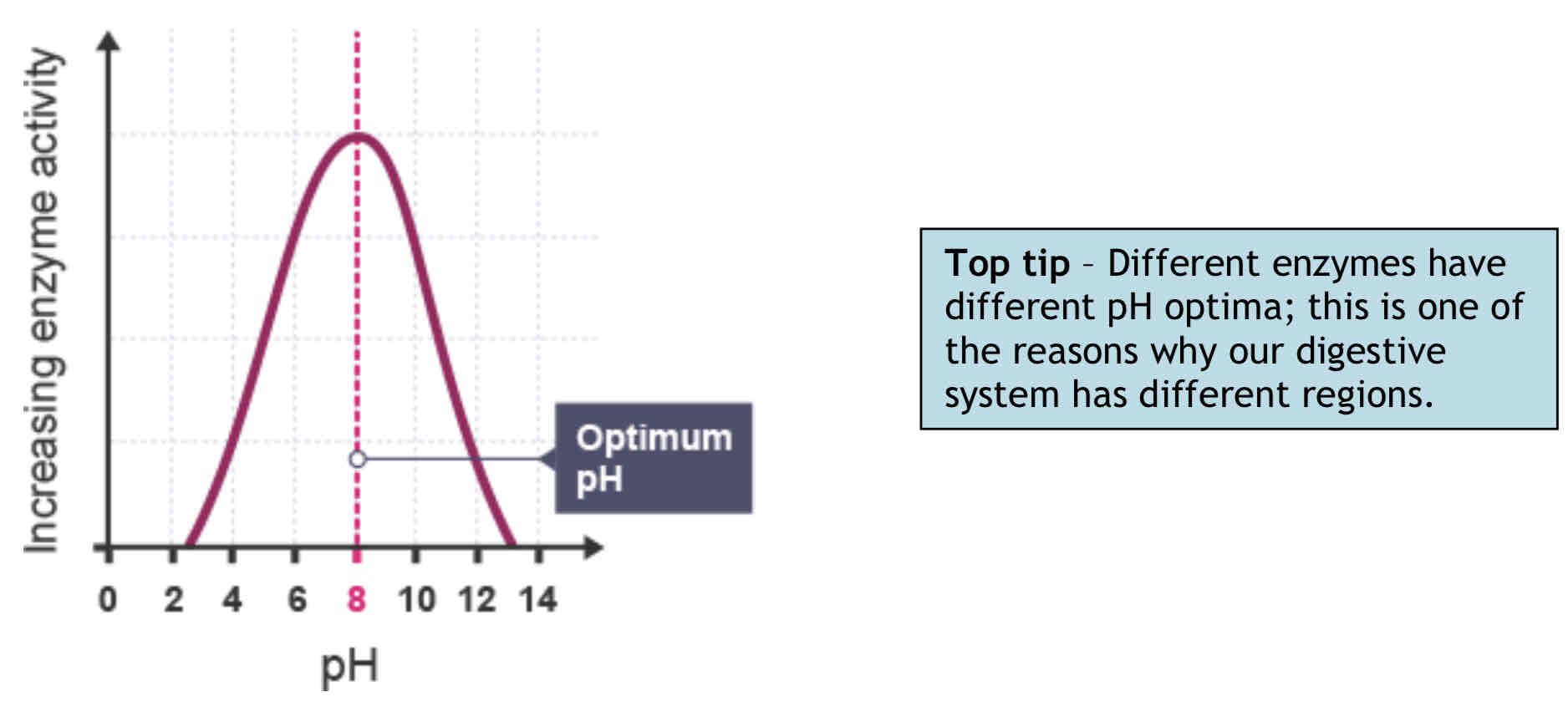
Factors affecting enzyme activity - pH
Enzymes have a narrow optimum pH range. Small changes in pH (within this range) can affect the rate of reaction without affecting enzyme structure.
Small changes outside the optimum range can cause reversible changes in enzyme structure; this results in inactivation.
Extremes of pH can denature an enzyme.
To form an enzyme-substrate complex the charges on the amino acid side-chains of the active site must attract charges on the substrate molecule.
The charges of the enzyme’s active site are affected by free hydrogen (H+) and hydroxyl (OH-) ions.
If, for example, there are too many H+ ions (too acidic) the active site and substrate may end up with the same charge. The enzyme active site and substrate would repel one another.
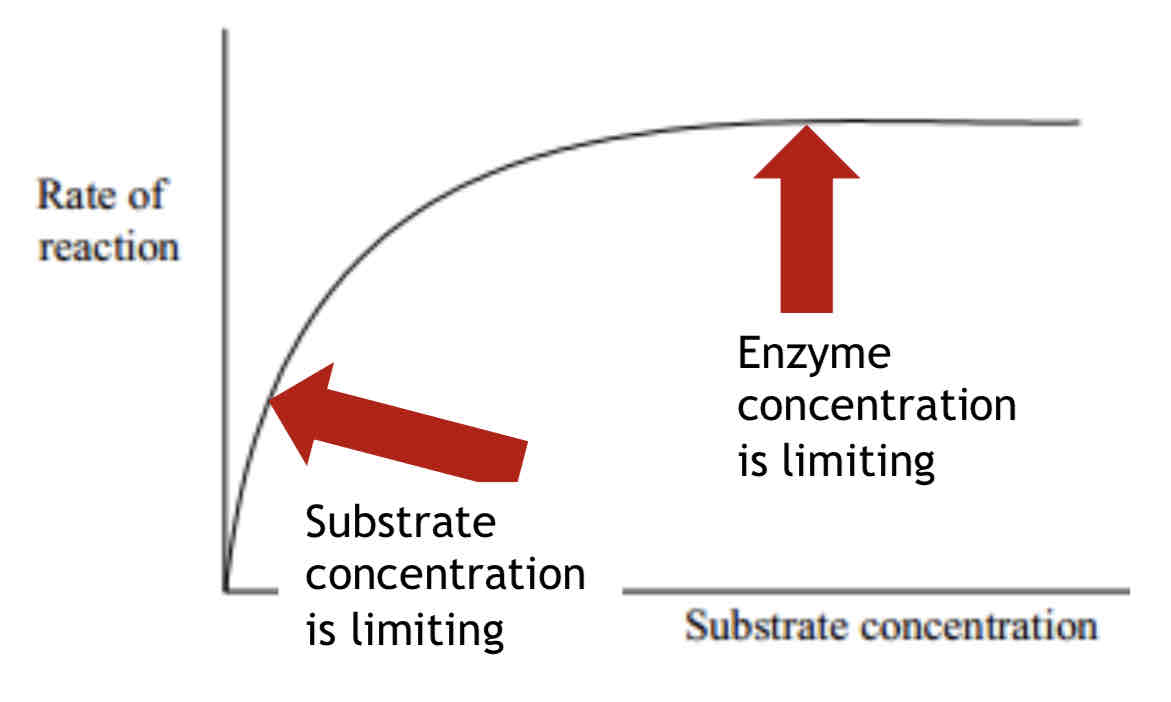
Factors affecting enzyme activity - Substrate Concentration
If the enzyme concentration remains constant, the rate of reaction will increase as the substrate concentration increases.
The reaction will level off once all the active sites are occupied; the number of available active sites becomes a limiting factor at higher substrate concentrations.
Initially the substrate concentration is the limiting factor.
As soon as the curve levels off the substrate is no longer limiting the rate of reaction; another factor becomes a limiting factor e.g. enzyme concentration.
When the enzyme concentration becomes a limiting factor all the active sites are occupied, having formed successful enzyme-substrate complexes.
Limiting factor – A factor is limiting when an increase in its value causes an increase in the rate of reaction.
Factors affecting enzyme activity - Enzyme Concentration
Once a product leaves the active site, the enzyme molecule can be re-used, so only a low enzyme concentration is needed to catalyse a large number of reactions.
The number of substrate molecules that one enzyme molecule can turn into products in a given time is called the turn-over number.
One of the fastest-acting enzymes is catalase found in all living cells, with a turnover number of 40 million molecules per second. Catalase breaks down the highly toxic waste, hydrogen peroxide into harmless water and oxygen.
As the enzyme concentration increases, there are more active sites available and therefore the rate of reaction increases.
If temperature and pH are optimal and there is an excess of substrate, the rate of reaction is directly proportional to the enzyme concentration.
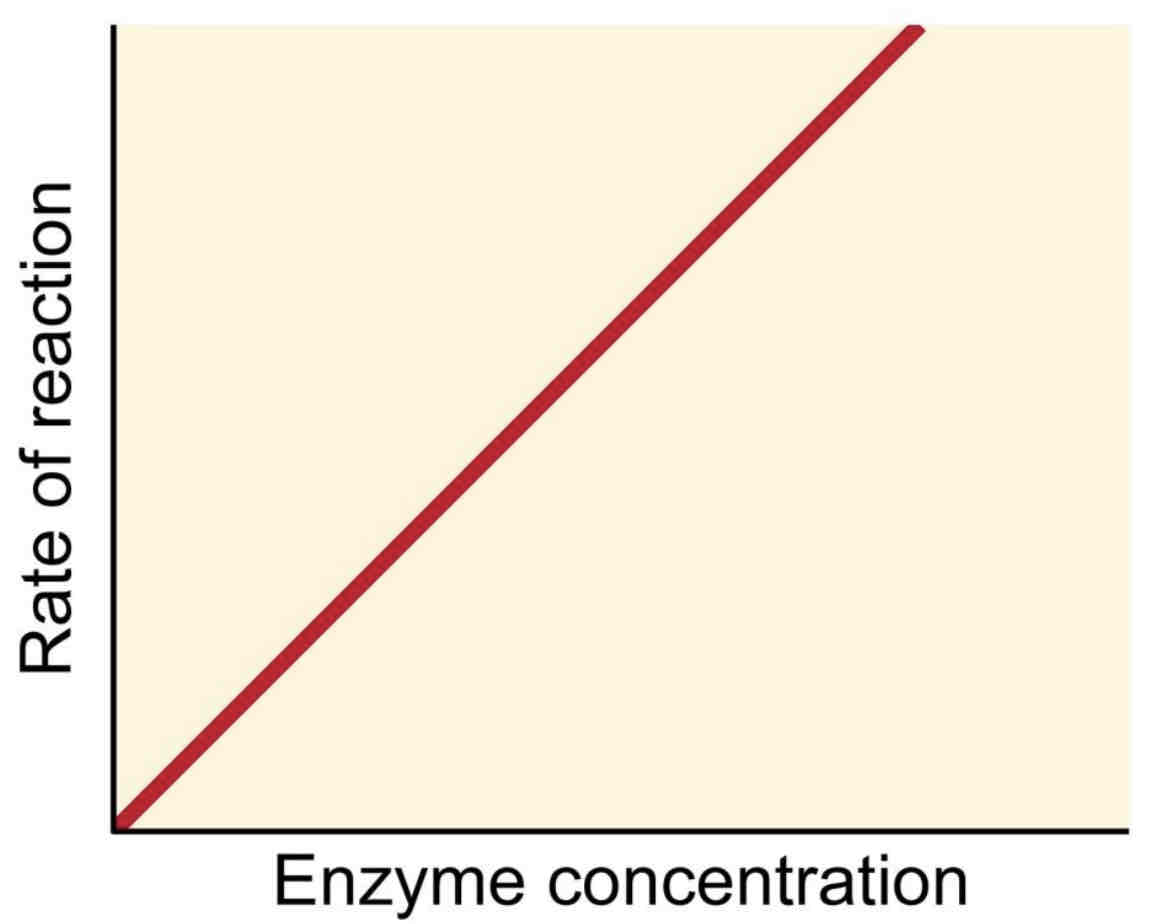
Why are Buffer Solutions used?
Buffer Solutions are used to maintain pH.
Why would it be inappropriate to use a buffer solution in an experiment on the hydrolysis of a triglyceride using Lipase?
Lipase changes the colour of the indicator as the pH changes.
This is because the lipase breaks down the triglyceride into fatty acids and glycerol.
The fatty acid lowers the pH until the solution becomes colourless.
However, adding the buffer would maintain the pH so would stop the pH from changing
What are Intra-cellular and Extra-cellular Enzymes?
Intra-cellular enzyme acts inside the cell.
Extra-cellular enzyme acts outside the cell.
What is an Enzyme Inhibitor?
An enzyme inhibitor is any substance which decreases the rate of an enzyme catalysed reaction or stops it.
Enzyme inhibitors are either competitive inhibitors or non- competitive inhibitors.
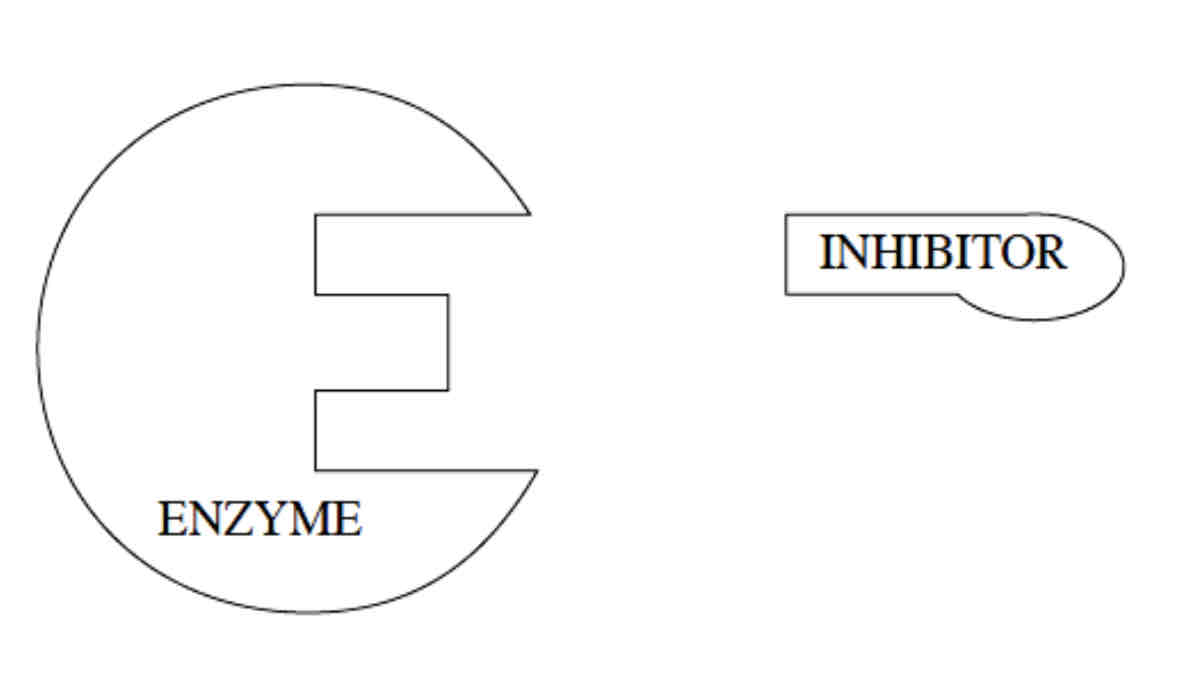
What are Competitive Enzyme-Inhibitors?
Competitive inhibitors are structurally similar to the substrate molecule; it has a complimentary shape and can fit in the active site instead of the substrate molecule. This forms an enzyme inhibitor complex. Therefore, it blocks the active site, preventing the substrate from attaching to the active site.
A competitive inhibitor prevents enzyme- substrate complexes forming.
There is less product formed, so, reducing the reaction rate.
Increasing the substrate concentration will decrease the effect of the inhibitor as the enzyme is more likely to collide with a substrate molecule and form a successful enzyme- substrate complex.
A benefit of competitive inhibitors is that they can prevent overproduction of end product/ harmful concentrations.
What are Non-Competitive Enzyme Inhibitors?
Non-competitive inhibitors do not bind to the active site; they bind to any other part of the enzyme (the allosteric site).
This alters the overall shape of the enzyme molecule, including the active site.
The substrate molecule can no longer fit into the active site. Successful enzyme-substrate complexes cannot form. (No catalysis can happen i.e no product is formed)
Increasing the substrate concentration will not increase the rate of reaction in this case as the substrate can no longer fit into the enzyme’s active site.
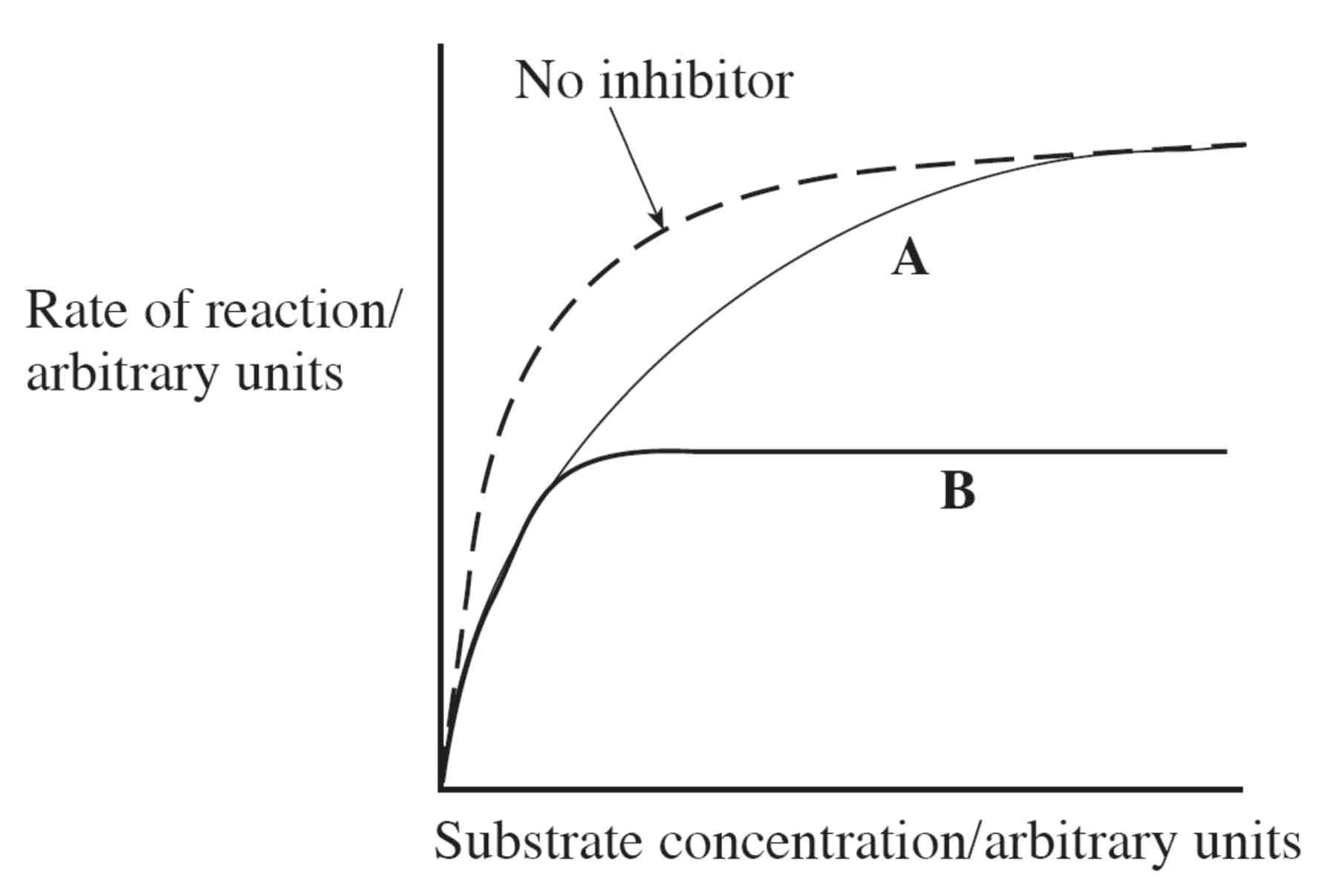
Identifying Competitive/ Non-Competitive Inhibitors from a Graph
The x-axis shows increasing substrate concentration.
Only competitive inhibition can be reduced by increasing the substrate concentration – so this must be curve A. - ends up with same amount of product.
Increasing the substrate concentration will not affect non-competitive inhibition – so this must be curve B.
How do you calculate the rate of reaction?
Change in Y/ Change in X
or
Rise/ Run
The Industrial Use of Enzymes - Immobilised Enzymes
Immobilised enzymes are fixed, bound or trapped on an inert matrix. An example is alginate beads.
Immobilised enzymes cannot move. This reduces the frequency of successful collision as the substrate is the only molecule moving.
Free enzymes will therefore always have greater activity provided the temperature is not greater than the optimum.
Enzymes Immobilised on a Membrane
Enzymes can also be immobilised on a membrane.
This is often preferable to using alginate beads as the enzyme can make direct contact with the substrate allowing the reaction to take place more quickly.
Substrate molecules must diffuse into the jelly matrix of alginate beads (the product also needs time to diffuse out), so the reaction takes longer.
Alginate beads may be used to immobilise lactase or pectinase.
Advantages of using Immobilised Enzymes
The enzyme does not contaminate the product.
The immobilised enzymes can be recovered and reused.
Only a small quantity of enzyme is needed.
The enzymes have greater stability and denature at higher temperatures.
Immobilised enzymes can catalyse reactions over a wider range of pH.
More than one enzyme can be used; enzymes can be added and removed.
Greater control over the process.
They can be used in a continuous process.
The breakdown of lactose
About three-quarters of the world’s human population are intolerant to lactose (milk sugar) in adulthood.
The lactose content of milk can be reduced by using the enzyme lactase.
Lactase breaks the disaccharide lactose into glucose and galactose.
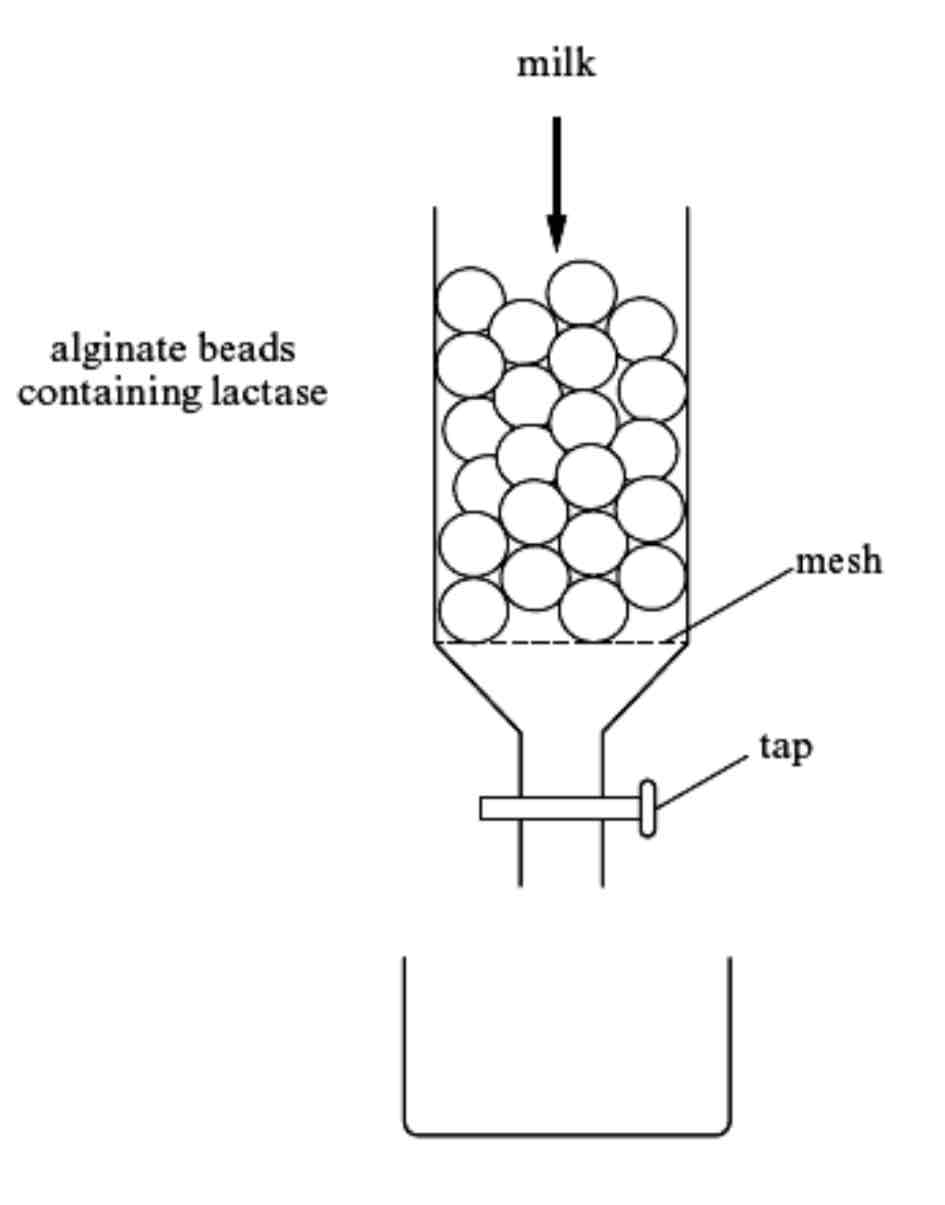
Describe what is happening in this diagram
As the milk flows through the column the substrate (lactose) diffuses into the alginate matrix and forms an enzyme-substrate complex with the lactase.
The monosaccharides glucose and galactose diffuse out of the alginate beads and leave the column with the rest of the milk.
Flow rate can be decreased to allow more contact time between enzyme and substrate, allowing more successful enzyme-substrate complexes to form.
Smaller beads can be used to increase the surface area allowing diffusion to take place quicker.
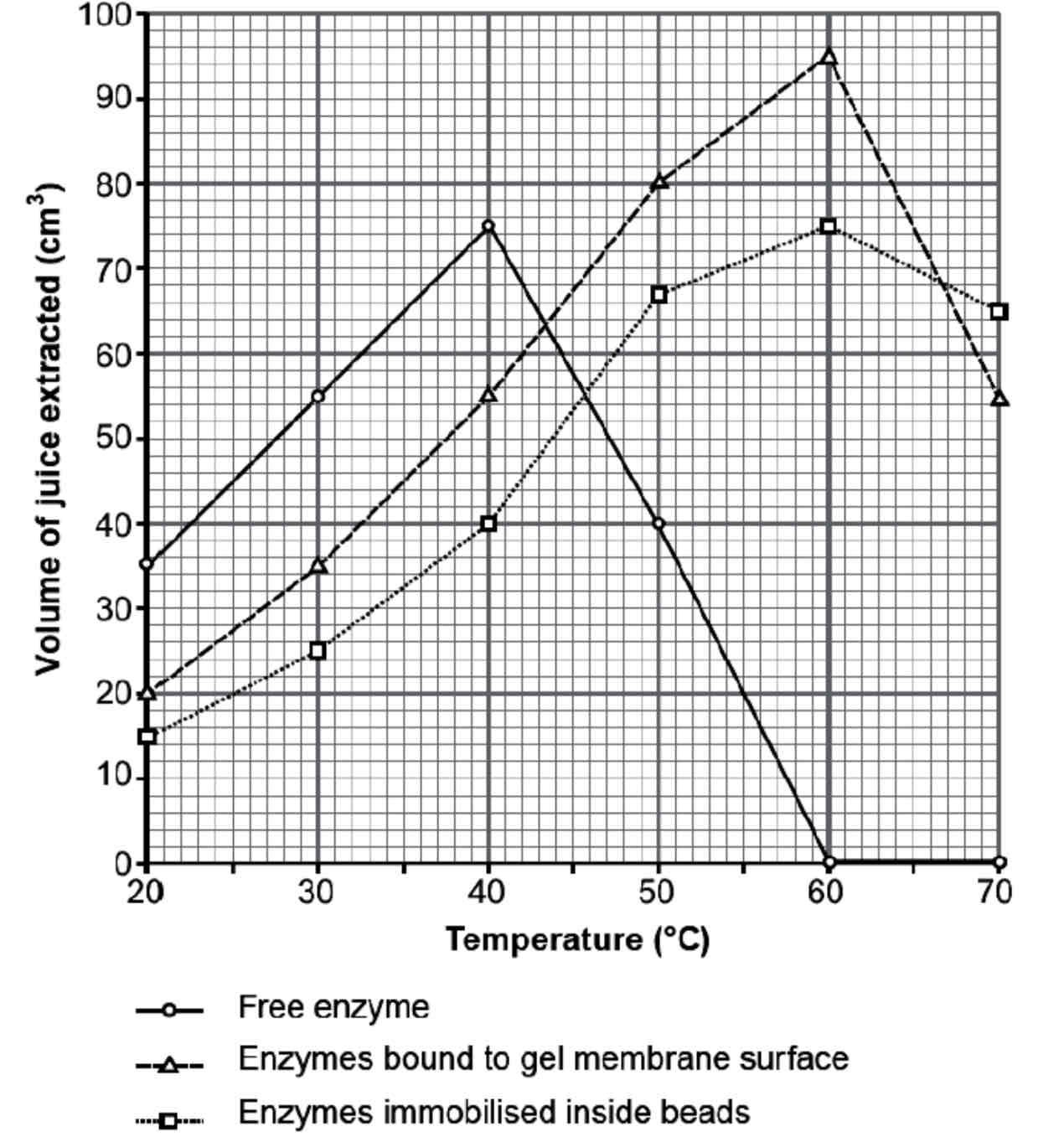
Describe & Explain the results of this graph for the free enzyme
Free enzyme - Between 20 – 40 oC the free enzyme has the greatest activity.
Both enzyme and substrate are free to move and are therefore more likely to collide.
As the temperature increases the kinetic energy of the molecules increases, allowing more successful collisions between enzyme and substrate and the product is produced quickly.
Between 40 – 60 oC the volume of fruit juice decreases sharply as increased vibrations break hydrogen bonds in the active site, this changes the shape of the active site and the enzymes become denatured.
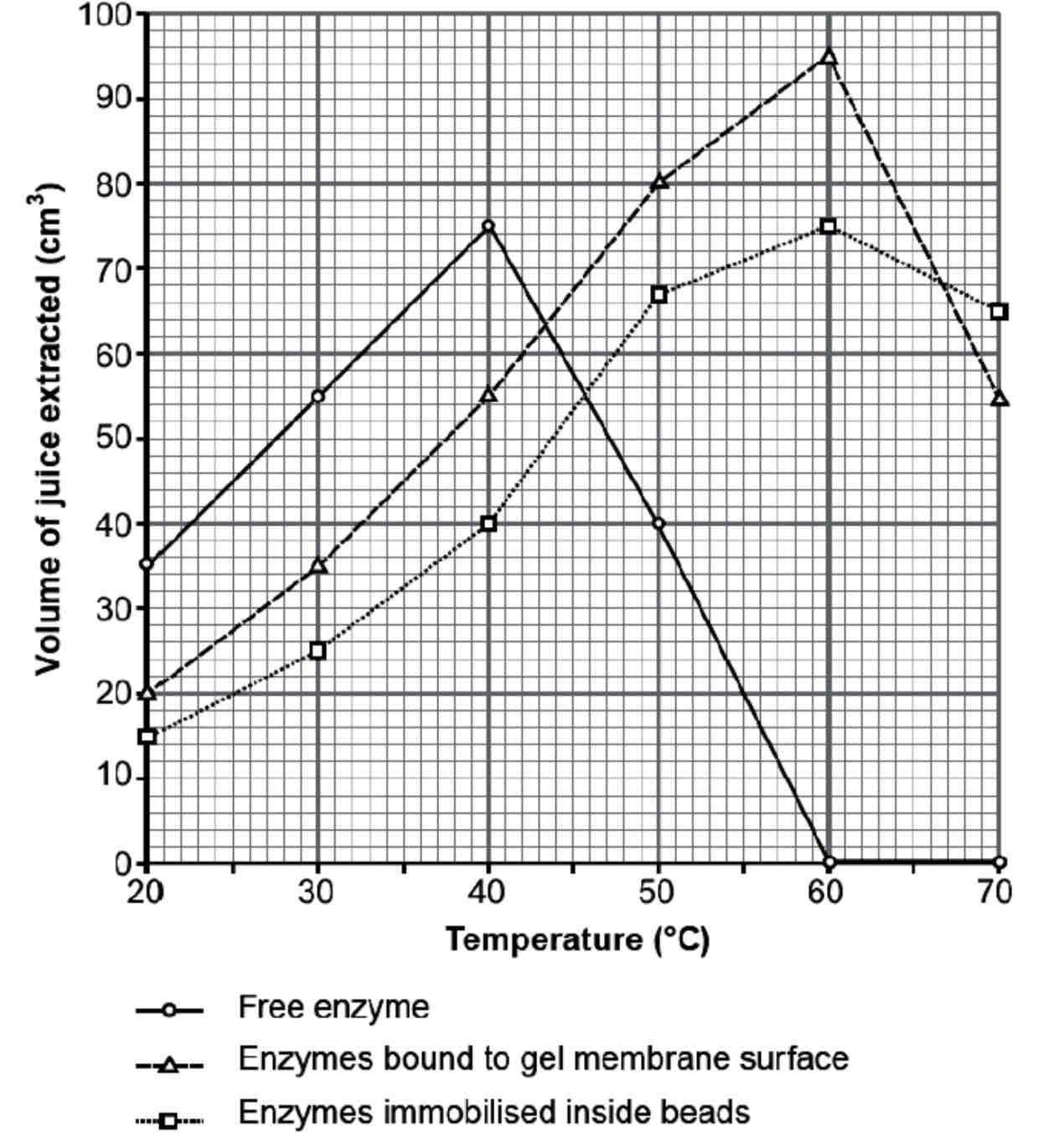
Describe & Explain the results of this graph for the Enzymes Immobilised inside beads & Enzymes bound to membrane
Enzyme immobilised in alginate beads – Enzyme activity continues to increase beyond the natural optimum (up to 60 oC).
The alginate gel fills and supports the enzyme’s active site, maintaining the shape of the active site, allowing enzyme-substrate complexes to continue to form.
Enzymes bound to a membrane – Membrane bound enzymes are in direct contact with the substrate and therefore the product is formed faster than with enzymes immobilised in alginate.
Which enzyme would give the greatest yield?
A fruit juice manufacturer would select the membrane bound method at 60 oC to produce the greatest yield of fruit juice.
To further increase yield, the membrane could be folded many times to increase the surface area and number of active sites available, the flow rate could also be reduced to allow longer contact time between enzyme and substrate.
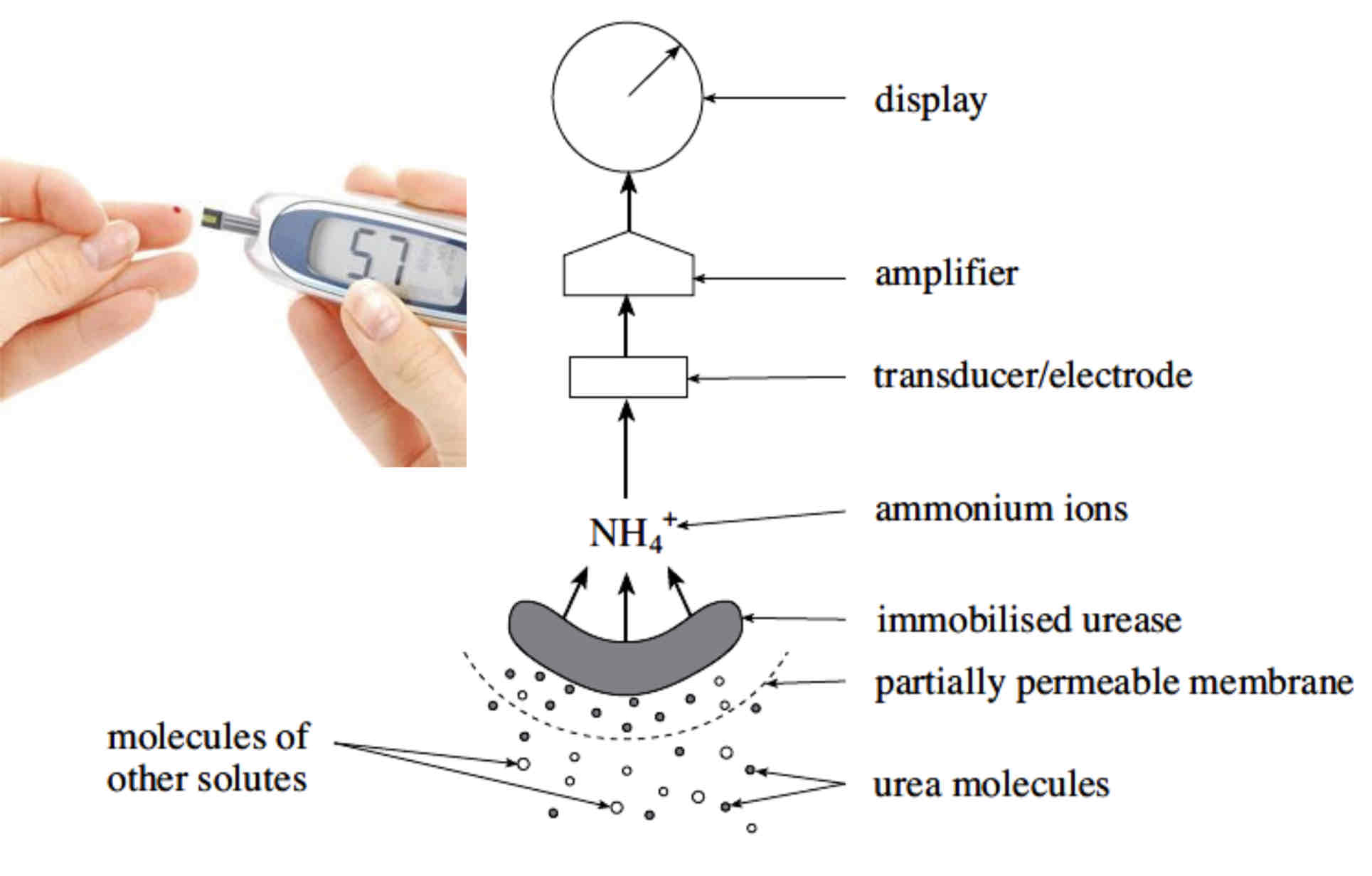
The Industrial Use of Enzymes - Biosensors
Biosensors can detect biologically important molecules very rapidly, even at low concentrations.
Biosensors can be used to measure blood glucose concentration in individuals suffering from diabetes.
Biosensors use immobilised enzymes on a gel membrane.
The biosensor detects a chemical change, as substrate is converted to product, and a transducer converts this chemical change into an electrical signal which can be amplified and viewed on a display.
Biosensor Diagram + Explanation
The biosensor above detects urea molecules. Small urea molecules diffuse across the partially permeable membrane and form enzyme-substrate complexes with immobilised urease.
The more Urea, the more ammonium ions produced/ relationship is directly proportional.
The product formed is ammonium ions (this is the chemical change); the transducer converts this into an electrical signal. The signal is amplified and reading is shown on the display.