Chem: investigation 9
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
What is an ideal gas?
An ideal gas is a theoretical concept that describes a gas following the ideal gas law.
What is the formula relating to Boyle's law?
P1V1=P2V2
What can change the properties and behavior of a gas?
1) number of particles
2) temperature
3) pressure
4) volume
Define Charles's law
This shows how gases expand when heat is applied, it states that at constant pressure the volume of a gas is directly proportional to the temperature.
Define Boyle's law.
A simple law that shows the behavior of a gas at constant temperature, it states that at constant temperature the volume of a gas is inversely proportional to pressure
What is the formula relating to Charles's law?
(V1/T1) = (V2/T2)
Define Gay-Lussac's law
It states that pressure is directly proportional to it’s temperature
What is the formula relating to Gay-Lussac's law?
(P1/T1) = (P2/T2)
What are some assumptions of the ideal gass law?
1) when the molecules collide they are elastic and have no friction, meaning there is no energy loss.
2) the molecules take up very little space, this implies they are far apart and have room to expand
3)there are no forces acting between the molecules or their surroundings
4) the molecules are always moving, preventing the gas to turn into a liquid at room temperature
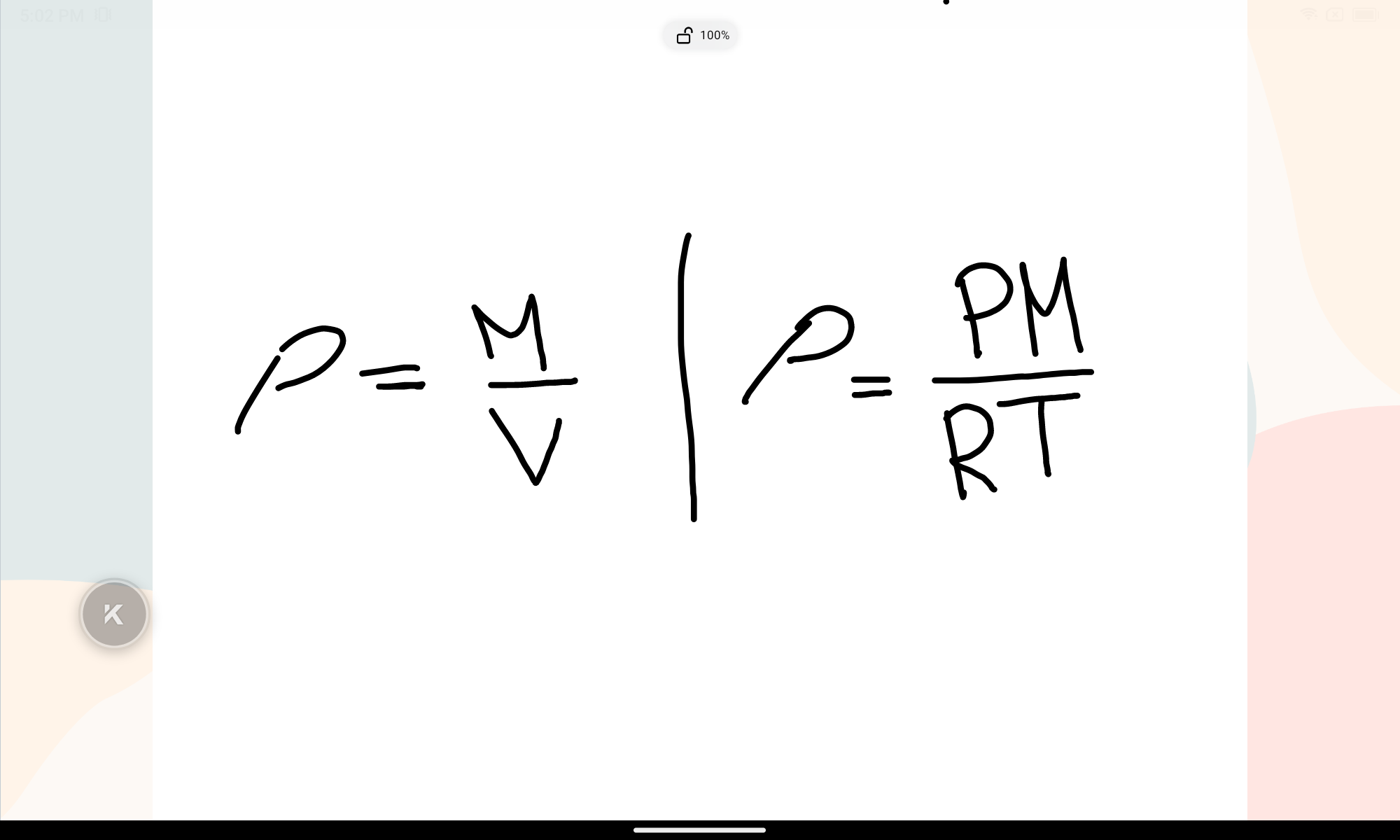
State the density formula
State the van der Waals equation
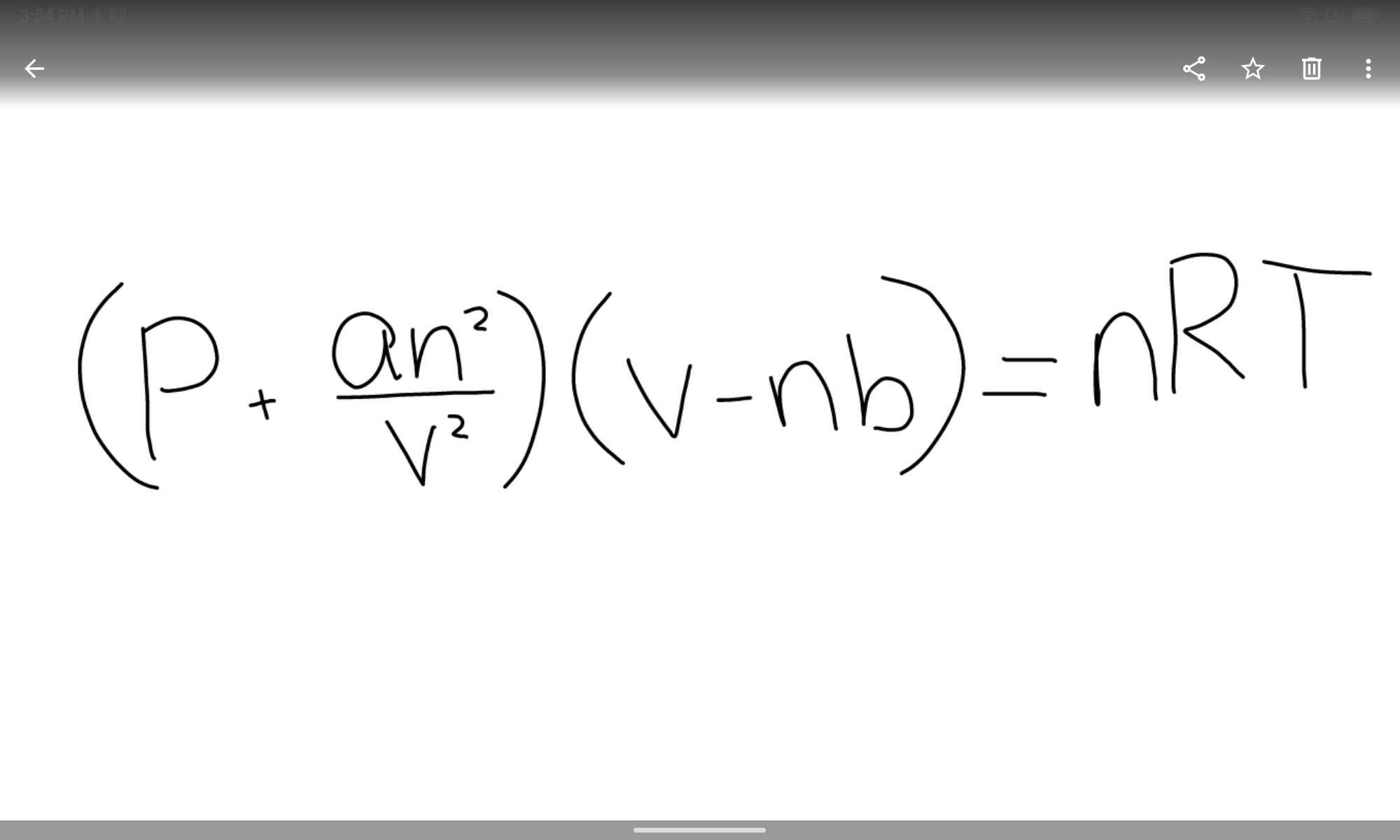
Define the isobaric process
A closed system process for which the pressure is held constant
Define the isovulumetric process
A closed system process for which the volume is held constant.
Define the isothermal process
A closed system process in which the temperature is held constant
Define absolute 0
The temperature at which all motion stops (-273°C) or (0K)
State the combined gas law
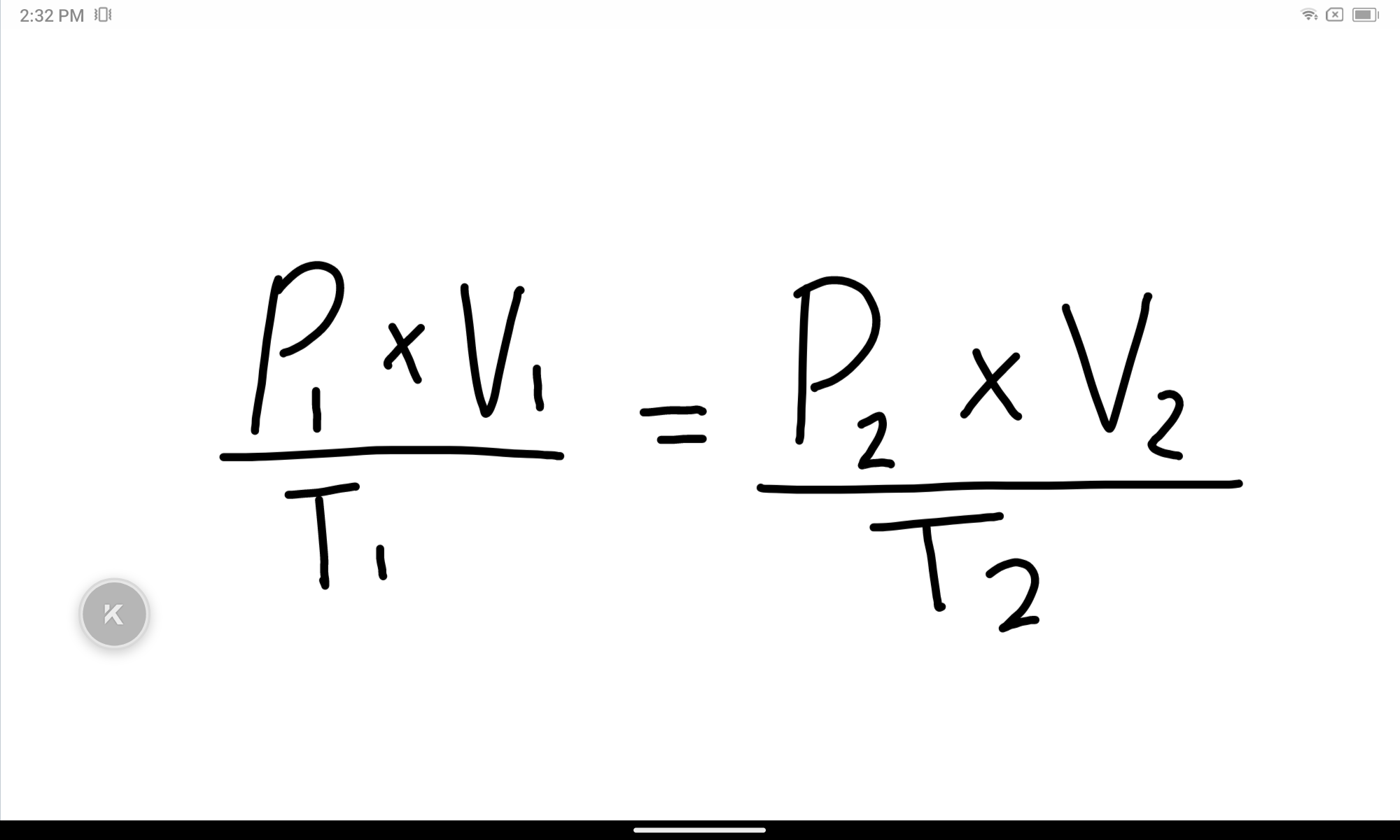
Define Avogadro's law
Avogadro's law states that and two samples of gas containing the same amount of moles will have the same volume when held at equal temperature and pressure