d and f block elements
1/74
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
What are transition elements?
A transition element is defined as the one which has incompletely filled d-orbitals in its ground state or in any one of its oxidation states.
What are d-block elements? Why are they known as transition elements?
These are the elements in which the last electron enters into the penultimate d orbital. Their general electronic configuration is (n-1)d1–10ns2. They are called transition elements because they form a bridge between the most reactive s-block metals and less reactive p-block metals.
List some properties of d-block elements
or
What are the characteristics of transition metals?
All d-block elements are metals - have a very hard crytsalline structure and high melting and boiling points
They have high tensile strength, malleability, and ductility
Generally paramagnetic in nature
They are electro positive with low ionisation energy
They show variable oxidation state
They show highest catalytic property and forms complex compounds easily
Zn, Cd, and Hg are not considered as transition elements. Why?
Atoms of these elements have completely filled d-orbitals in their ground state as well as in their common oxidation states, so they are not regarded as transition elements.
On what ground state can you say that scandium (Z = 21) is a transition metal but Zn (Z = 30) is not?
Scandium has an incomplete 3d orbital, but zinc has a completely filled d-orbital in both ground and oxidation states.
Silver has a completely filled d-orbital (4d10) in its ground state. How can you say it is a transition element?
Silver (Z = 47) can exhibit a +2 oxidation state where it will have an incompletely filled d-orbital, making it a transition metal.
Comment on the metallic character of transition metals.
They are hard metallic solids with low volatility.
Due to the greater effective nuclear charge and large number of unpaired electrons in d-orbital, they have strong metallic bonds.
Transition metals and many of their compounds show paramagnetic behaviour. Explain
In the case of transition metals, as they contain unpaired electrons in the (n-1) d-orbitals, most of the transition metal ions and their compounds are paramagnetic.
The transition metals (with the exception of Zn, Cd and Hg) are hard and have high melting and boiling points. Why?
or
Transition metals are much harder than the alkali metals. Why?
Due to the presence of unpaired electrons, transition metals have a strong metallic bond.
Scandium forms no coloured ions, yet it is regarded as a transition elements. Explain why?
Sc in the ground state has one electron in the 3d-subshell. Hence, it is regarded as a transition element. However, in its common oxidation state +3, it has no electron in 3d subshell. Hence, it does not form coloured ion.
Why do Mn and Tc have low melting points?
They have stable half- filled d-orbitals, hence the electrons are tightly held by the nucleus, due to which there is a weak covalent and metallic bond.
Zn, Cd, and Hg have the lowest melting point in their respective series. Why?
They have completely filled d-orbitals, leading to weak metallic bonds and no covalent bonding.
Which element in 3d series has the highest melting and boiling points?
Cr because it has a stable 3d54s1 config with the highest number of unpaired electrons and hence has a greater metallic bond strength
Transition metals exhibit high enthalpy of atomization. Why?
Because of a large number of unpaired electrons, they have strong interatomic interactions.
Mn and Tc, similarly Zn, Cd, and Hg, have the lowest enthalpy of atomization. Why?
Mn and Tc have stable half-filled d-orbitals, while Zn, Cd, and Hg have completely filled d-orbitals, leading to weak bonding.
Atomic and ionic radii vary along the period and down the group. Explain the trends.
In a period, from left to right, with increase in the atomic number, the atomic radius decreases due to increase in the nuclear charge which increases the attraction of the nucleus for the valence electrons. Down the group, atomic radius increases due to additional shells.
5d and 4d transition elements have almost the same size. Why?
This is due to lanthanoid contraction as a result of ineffective shielding by 4f electrons.
Ionization energy increases in a particular series. Why?
This is due to the increase in effective nuclear charge, which decreases atomic size and increases ionization energy.
First ionization enthalpy of Cr and Cu is low. Why?
Removing the first electron gives stable 3d5 and 3d10 configurations, respectively.
Second ionization enthalpy of Cr and Cu is higher than those of neighbouring elements. Why?
After the first ionization, stable 3d5 and 3d10 configurations resist further electron removal.
The increase in ionization energy along the period of d-block elements is very small. Why?
The increase in nuclear charge is partially cancelled by the screening effect of d-orbitals.
The 2nd ionization enthalpy is low for Mn and Zn but the 3rd ionization enthalpy is high for Mn and Zn. Why?
The 2nd ionization gives stable d5 and d10 configurations, making the 3rd ionization harder.
5d transition elements possess higher ionization energy than 3d and 4d elements. Why?
This is due to greater nuclear charge and ineffective shielding by 4f electrons.
Why are Ni2+ compounds thermodynamically more stable than Pt2+ compounds?
The sum of the first two ionization energies is lower for Ni than for Pt.
Cu has a positive standard electrode potential. Why? Can it liberate H2 from acids?
Cu has high atomization enthalpy, low hydration enthalpy, and high ionization energy, resulting in a positive potential. It cannot displace H2 from acids.
Ni has a more negative electrode potential than expected. Explain.
Nickel has relatively higher hydration enthalpy or more negative hydration enthalpy
Mn has a smaller E0 value than expected. Explain
Due to presence of stable d5 configuration after losing 2 electrons (low IE2)
Zn has a smaller E0 value than expected. Explain
Due to presence of stable d10 configuration after losing 2 electrons (low IE2)
Why do transition elements show variable oxidation states?
Due to less energy difference between ns and (n-1)d orbitals, transition elements can use their ns and (n – 1)d orbital electrons for bond formation therefore, they show variable oxidation states.
d-block elements exhibit more oxidation states than f-block elements. Explain.
Because of smaller energy gap between (n-1) d and ns orbitals both (n-1)d and ns electrons involve in bonding in d-block elements, while relatively energy gap between (n-2)f and ns electrons are greater in f-block elements.
Explain the trend in oxidation states of transition metals.
Transition metals show variable oxidation states due to ns and (n-1)d electron participation, with lower oxidation states involving ns electrons and higher states involving both.
Cu+ ions are unstable in aqueous solutions. Why?
Cu+ ions are disproportionate to Cu2+ and Cu due to the greater hydration enthalpy of Cu2+.
Cu+ → Cu2+ + Cu
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
Mn2+ has a stable half-filled d5 configuration, whereas Fe2+ can easily lose an electron to attain stable d5.
With the same d-orbital configuration (d4), Cr 2+ is a reducing agent while Mn 3+ is an oxidising agent. Explain
Cr2+ losing one electron becomes Cr3+ gains half filled t2g3 configuration
Mn3+ gaining one electron becomes Mn2+ gains a half filled d5 configuration.
Co2+ is easily oxidised to Co3+ in the presence of a strong ligand. Explain.
In the presence of a weak ligand, Co²⁺ (d⁷) has the electronic configuration t₂g⁵ eₙ², i.e., there are three unpaired electrons.
But in the presence of a strong ligand, the difference in energy between t₂g and eₙ levels increases.
As a result, out of the three unpaired electrons, two become paired, and there remains only one unpaired electron in the eₙ level.
This electron from the higher energy eₙ level is easily lost to form the Co³⁺ ion, having a stable configuration t₂g⁶ with high CFSE (Crystal Field Stabilization Energy).
Thus, Co²⁺ is easily oxidized to Co³⁺ in the presence of a strong ligand.
Why do transition metals form coloured compounds?
Due to presence of unpaired electrons, d-d transition takes place.
Why is the E° value of Mn3+/Mn2+ much more positive than Cr3+/Cr2+ or Fe3+/Fe2+?
The third ionization energy of Mn is very high due to its half-filled d5 configuration, making Mn3+ a strong oxidizing agent with a positive E° value.
Explain the trend in the stabilities of lower and higher E° values of Sc3+/Sc2+, Zn3+/Zn2+, and Fe3+/Fe2+.
Sc3+ has a stable noble gas configuration (no tendency for reduction). Zn3+ tends to accept one electron to attain a stable configuration. Fe3+ is stable due to its half-filled d5 configuration.
What is meant by disproportionation of oxidation states?
When an oxidation state decomposes to form ions of higher and lower oxidation states, it's called disproportionation. Example: MnO4²⁻ + 4H⁺ → MnO4⁻ + MnO₂ + H₂O.
Among the transition series, which element shows the highest oxidation number?
Mn (+7) in the 3d series, Ru (Ruthenium) (+8) in the 4d series, and Os (Osmium) (+8) in the 5d series.
Sc does not show +2 oxidation state. Explain
Sc has 3d14s2 configuration and by losing 3 electrons, Sc gains noble has configuration
Mn has maximum number of oxidation states. Explain.
Mn has 3d54s2 configuration. Hence it can show oxidation states ranging between +2 and +7
The only oxidation state of Zn is +2. Why?
Zinc has 3d104s2 config and by losing 2 electrons it has a stable d10 configuration, and its 3d electrons are not involved in bonding.
Why is the highest oxidation state of a metal exhibited in its oxide and fluoride only?
Oxygen and fluorine have small sizes and high electronegativities, enabling metals to achieve their highest oxidation states.
Why Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4?
Manganese can form pπ - dπ bond with oxygen by utilising 2p-orbital of oxygen and 3d-orbital of manganese due to which it can show highest oxidation state of +7. While with fluorine it cannot form such pπ - dπ bond thus, it can show a maximum of +4 oxidation state.
Why do most transition metals not show a +1 oxidation state?
The +1 oxidation state is unstable and undergoes disproportionation. Example: Cu⁺ → Cu²⁺ + Cu.
Arrange the MnO−4,Cr2O2−7 and VO2+ in the increasing order of their oxidising power.
Why do transition metal ions and compounds act as good catalysts?
d-block elements act as very good catalysts due to presence of more vacant d-orbitals, can exhibit variable oxidation states, and easily form intermediate complexes, thereby reducing the activation energy of reactions.
Which metal in the first transition series exhibits the +1 oxidation state most frequently?
Copper, due to its stable d10 configuration in the +1 oxidation state.
Name some colourless ions
Cu+, Zn2+, Sc3+, Ti4+
Predict which ions are coloured in an aqueous solution: V3+, Mn2+, Fe3+, Ti3+, Co2+, Sc3+, Cu+.
Except Sc3+ and Cu+, all others are coloured due to partially filled d-orbitals.
What are interstitial compounds? What are their properties?
The compounds formed when small atoms of H, C or N get trapped inside the crystal lattice of metals is known as interstitial compounds
Properties:
High melting point; higher than pure metals
Very hard; some borides approach diamond in hardness
Retain metallic conductivity
Chemically inert
Can d-block elements easily for alloys? Justify.
Yes, they form alloys easily due to similar atomic radii. These elements can replace one another in a metallic crystal lattice.
Ex: Bronze(Copper-Tin), Brass (Copper-Zinc)
Why does KMnO4 act as an oxidizing agent in neutral and faintly alkaline medium?
KMnO4 oxidizes iodides to iodates and manganous salts to MnO2 due to its Mn in a high oxidation state (+7).
What is the effect of heat on KMnO4?
or
Upon heating, it transforms into potassium manganate (K2MnO4), which is green in color.
It decomposes to form potassium manganate (K2MnO4) which is green in colour and MnO2, and O2.
2KMnO4(s)→K2MnO4(s)+MnO2(s)+O2(g)
KMnO4 acts as an oxidising agent in acidic medium. Write the ionic equations to support this
KMnO₄ is a good oxidizing agent in acidic, basic, and neutral media. The oxidizing action in acidic medium is due to the reaction:
MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
Why does the color of sodium chromate solution changes on passing CO2 gas under pressure? Write the chemical equation and the colour of various species?
Why the addition of sodium sulphite does turn acidified K2Cr2O7 solution green?
The yellow coloured sodium chromate solution will changes to orange red when CO2 passed through it. CO2 makes the medium acidic and lowers the pH. In acidic pH, chromate becomes dichromate and colour changes.
2CrO42- (yellow) + 2H+→ Cr2O72- (orange) + H2O
Sodium sulphite (Na2SO3) reduces acidified K2Cr2O7 to chromic sulphate which is green in colour.(Cr3+ is green in colour)
Why are permanganate titrations not carried out in the presence of HCl?
HCl is oxidized to chlorine gas, affecting the accuracy of the titration.
What is the preparation method of potassium dichromate (K2Cr2O7)?
Potassium dichromate is prepared by fusing chromite ore (FeCr2O4) with Na2CO3 and oxygen, followed by acidification and treatment with KCl.
Why is only dilute sulphuric acid used for acidifying KMnO4 not dil.HCl or HNO3 ?
HCI gets oxidized to Cl2, Whereas HNO3 itself is a strong oxidizing agent and hence oxidises the reducing agent.
Name a member of the lanthanoid series which is well known to exhibit +2 oxidation state. Why?
Eu (Europium) - Due to the 4f7 6s2 configuration losing 2 electrons Eu gets half filled 4f7 configuration.
Yb (Ytterbium) - Due to the 4f14 6s2 configuration losing 2 electrons Yb gets fully filled 4f14 configuration.
Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state. Why?
Ce (Cerium) - Due to the 4f15d16s2 configuration losing 4 electrons Ce gets a noble gas configuration.
Elements like Pr (Praseodymium), Nd (Neodymium), Tb (Terbium), and Dy(Dysprosium) also show +4
What is trans — uranium elements?
Most of the elements in actinides are artificially prepared and are short lived. They are radioactive. The elements after uranium is artificially prepared and so they are called trans-uranium or trans-uranic elements
Among ionic species, Sc3+, Ce4+ and Eu2+, which one is a good oxidising agent?
Yb2+ is a good reductant.
Tb4+ is an oxidant.
Ce4+; The stable oxidation state of lanthanoids is +3. Ce4+ tends to accept an electron to change to a +3 state. Hence, it acts as a good oxidising agent.
A reducing agent undergoes oxidation, Yb2+ tends to oxidise to its more stable +3 state and hence acts as a reductant.
An oxidising agent undergoes reduction, Tb4+ tends to reduce to its more stable +3 state and hence act as an oxidant.
Ce(IV) is a good analytical reagent.
The cerium (IV) has sufficient kinetic stability due to the reaction rate being very slow and hence cerium (IV) is a good analytical reagent.
Eu(III) is easily reduced to Eu(II). Explain.
Due to electronic configuration stability, europium prefers to exist as Eu²⁺ instead of Eu³⁺ .
Eu: [Xe] 4f76s2
Eu³⁺: [Xe] 4f⁶ (partially filled f-orbital, unstable)
Eu²⁺: [Xe] 4f⁷ (half-filled f-orbital, highly stable)
A half-filled f⁷ configuration provides extra stability due to exchange energy, making Eu³⁺ more likely to gain an electron and form Eu²⁺.
Why do actinoids show a wide range of oxidation states then the corresponding members in the lanthanoid series?
Actinides exhibit larger oxidation states than lanthanides, because of the very small energy gap between 5f, 6d and 7s subshells
What is lanthanoid contraction, and what are its consequences?
Lanthanoid contraction is the steady decrease in size across the lanthanoid series due to poor shielding of 4f orbitals. Consequences include
4d and 5d series elements show similar atomic radii and physical properties
Difficult to separate lanthanoids
Similar atomic sizes of Zr and Hf
Reduced basicity of lanthanide hydroxides from Lanthanum to Lutitium with increase in atomic number
Give reason: La(OH)₃ is a stronger base than Lu(OH)₃
or
Basicity decreases from La(OH)₃ to Lu(OH)₃. Why?
This is due to lanthanide contraction. As the size of the lanthanide ions decreases from La³⁺ to Lu³⁺, the covalent character of hydroxides increases with the decrease in size, and hence, basic strength decreases.
Therefore,
La(OH)₃ is most basic.
Lu(OH)₃ is least basic.
What is actinoid contraction?
The steady decrease in ionic size across the actinoid series, caused by ineffective shielding of 5f orbitals.
Why is actinoid contraction greater than lanthanoid contraction?
or
Lower ionisation enthalpy of early actinoids as compared to the early lanthanoids. Explain.
5f orbitals are more diffused and provide less shielding than 4f orbitals, resulting in a stronger nuclear charge.
Write some differences between lanthanoids and actinoids.
Lanthanoids are mostly non-radioactive, exhibit +3 oxidation states predominantly, and form fewer complexes. Actinoids are radioactive, exhibit a broader range of oxidation states (+3 to +7), and form more complexes.
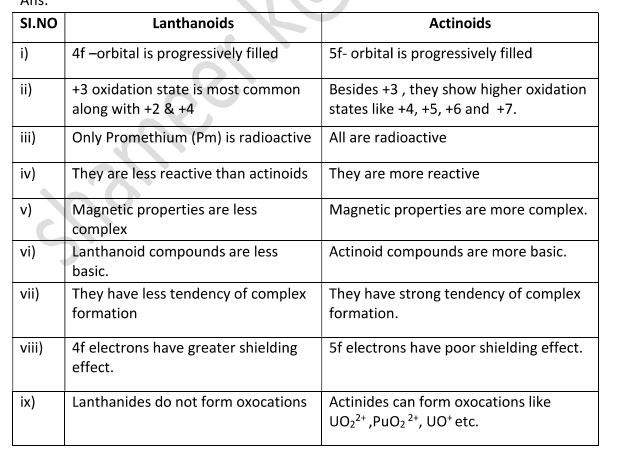
Actinoids have more tendencies to form complexes than lanthanoids. Why?
Since lanthanoids have less charge density their complexing ability is less. Actinoids have more tendencies to form complexes due to high charge density (small size and high nuclear charge).
Name an important alloy which contains some of the lanthanoid metals. Mention its two uses.
Mischmetal is a well known alloy which consists of a lanthanoid metal (about 95%), iron (about 5%) and traces of S, C, Ca, Al etc.
Mischmetal is used in Mg based alloy to produce bullet shells and lighter flint.
List applications of d- and f-block elements.
TiO2 is used in pigments, MnO2 in dry batteries, AgBr in photography, molybdenum in X-ray tubes, and Ziegler-Natta catalyst in polymer production.