IB chemistry: topic 2: electron shells and orbitals
0.0(0)
0.0(0)
Card Sorting
1/27
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
1
New cards
what was Bohr Theory?
the atom consisted of a small nucleus of protons and electrons surrounded by circulating electrons
2
New cards
what is an isotope?
forms of an element with the same number of protons but different number of neutrons - results in different physical properties
3
New cards
what is true of isotopes?
same chemical properties, same atomic number, different physical properties
4
New cards
what is a shell?
a group of atomic orbitals with the same principal quantum number (n)
5
New cards
what is "n"?
principal quantum number
6
New cards
what does the principal quantum number indicate?
the shell that the electrons occupy
7
New cards
what is the maximum number of electrons that can occupy a shell?
2n²
8
New cards
what does the principal quantum number represent?
the overall energy of each orbital, which increases the further the shell is from the nucleus
9
New cards
what is an orbital?
a region of space where one is likely to find an electron
10
New cards
what is an example of Heinsberg uncertainty principle?
orbitals
11
New cards
what are characteristics of orbitals?
can hold up to 2 electrons as long as they have opposite spin
12
New cards
what is an example of Pauli's exclusion principle?
2 electrons held in orbitals that have opposite spin
13
New cards
what are the four orbitals?
s, p, d, f
14
New cards
what shape is "s" orbital?
spherical
15
New cards
what shape is "p" orbital?
dumbbell
16
New cards
what shape is "d" orbital?
various
17
New cards
what shape is "f" orbital?
various
18
New cards
what is the occurrence of "s" orbitals?
one in every principal level
19
New cards
what is the occurrence of "p" orbitals?
three in levels 2 upwards
20
New cards
what is the occurrence of "d" orbitals?
five in levels 3 upwards
21
New cards
what is the occurrence of "f" orbitals?
seven in levels 4 upwards
22
New cards
why are orbitals not filled in numerical order?
as the principal energy levels get closer together as you get further from the nucleus, resulting in overlaps in sub levels
23
New cards
what is the first example of a sub level overlap?
4s orbital is filled before the 3d orbitals
24
New cards
what are elements that do not follow the orbital filling rule?
chromium and copper
25
New cards
how does chromium's orbitals fill up?
3d fills up so it is semi full, 3d⁵, leaving 4s as 4s¹
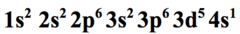
26
New cards
how does copper's orbitals fill up?
3d fills up to it is full, 3d¹⁰, leaving 4s as 4s¹
27
New cards
why do copper and chromium have unique electron configurations?
it is much more stable to fill up (semi or full) their 3d orbitals
28
New cards
when forming ions, how do the electrons leave the shells?
electrons are removed first from the highest occupied orbital, 4s THEN 3d