Thermodynamics 3
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
what is the 3rd law of thermodynamics
the entropy of all pure crystalline solids at the absolute zero of temperature is zero
how do you calculate an entropy value at a given temprature
consider the various entropy changes as he temprature is raised from 0K
what two phase changes does the 3rd law of thermodynamics go under
melting
vaporisation
what system can take account of enthalpy change and entropy change
free energy
what is the gibbs free energy formula
G = H - TS
what is gibbs free energy
shows the balance between enthalpy and entropy terms in determing the direction of a process
whats the expression for standard state changes between products and reactants
triangleG = triangleH - TtriangleS
what will happen to the process if is the change in value of gibbs free energy is less than 0
it will occur
what will happen to the process if the chnage in value in free energy is greater than 0
process will occur in reverse direction
what will happen to the process if the chnage in value in free energy is 0
reversible
what is the value of the change in energy when a reaction is at equilibrium
0
what is chemical potential
the contribution that component makes to the overall free energy of a system
whats a simple definition for a pure material
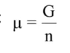
what is an activity
enable us to quantify how the chemical potential of a pure substance changes when it becomes a component in a mixture
when temprature is increased what happens to the internal energy
also increases