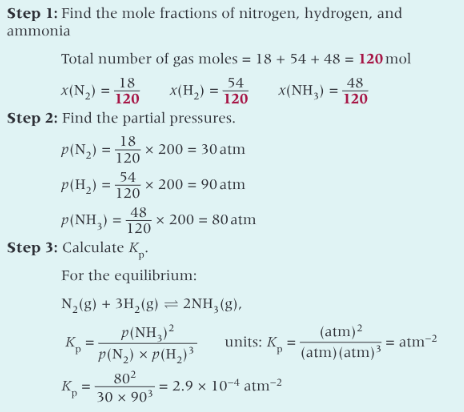19.2 the equilibrium constant Kp
0.0(0)
0.0(0)
New
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
mole fraction of a gas
its proportion by volume to the total volume of gases in a gas mixture
mole fraction(a) = number of moles of A/ total number of moles in a gas mixture
2
New cards
partial pressure of a gas in a gas mixture
the contribution that the gas makes towards the total pressure P. The sum of the partial pressures of each gas equals the total pressure
partial pressure p(a) = mole fraction of A x total pressure (p)
3
New cards
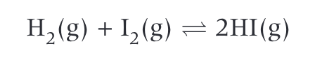
write equation for the partial pressure of:
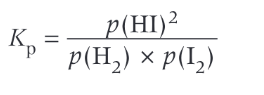
4
New cards
example of units for Kp
pascals
kilopascals
atmospheres
5
New cards
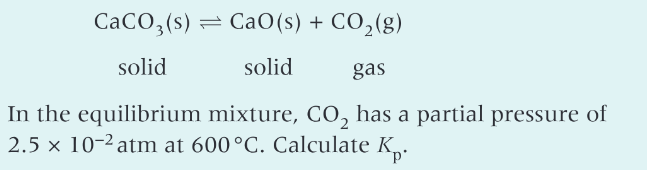

6
New cards