elsaid - cancer immunotherapy
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
cell-based immune response against tumors
concept of immune system activated by antigens presented on surface of cancer cells
dendritic cells (DC) are professional antigen presenting cells and when they encounter cancer cells with antigens presented on their surface, they take up the tumor antigens
dendritic cells enter lymphatic tissues and mature —> mature DCs present antigens to T-cells
activated T-cells leave lymph nodes and migrate to tumor bearing antigens to destroy tumor cells
mechanisms of immune evasion by tumors
tumors acquire immune tolerance
early in tumorigenesis, tumor cells can be classified as highly immunogenic or poorly immunogenic
immune response (innate and adaptive) removes the highly immunogenic cells and the remaining poorly immunogenic cells evade the immune system by:
downregulating genes encoding for proteins involved in antigen presentation on tumor cells
upregulating genes encoding for proteins that provide an immunosuppressive environment e.g. TFG-beta, IL-10
types of tumor antigens
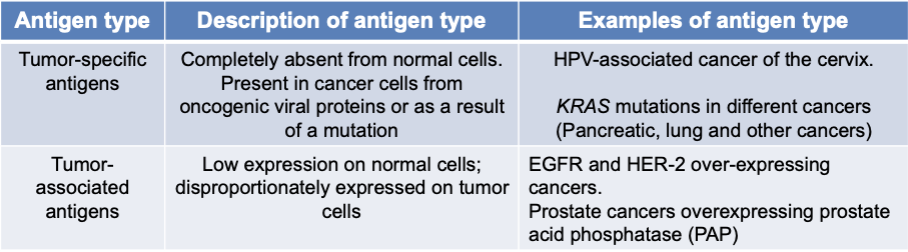
tumor-specific antigens
completely absent from normal cells
present in cancer cells from oncogenic viral proteins or as a result of a mutation
examples:
HPV-associated cancer of the cervix
KRAS mutations in different cancers (pancreatic, lung, and other cancers)
tumor-associated antigens
low expression on normal cells
disproportionately expressed on tumor cells
examples:
EGFR and HER-2 over-expressing cancers
prostate cancers overexpressing prostate acid phosphate (PAP)
classification of cancer immunotherapy
immune checkpoint inhibitors
monoclonal antibodies against cell surface markers
cell-based immunotherapy (adoptive cell transfer)
cytokines
immune checkpoint: CTLA4
CTLA-4 is an immune checkpoint that prevents excessive stimulation of effector T-cells
effector T-cells express a number of co-stimulatory and co-inhibitor receptors
CTLA4 is a co-inhibitory receptor on T-cells
signal 1 (priming) is initiated when TAAs attached to MHC on APCs bind to the TCR
signal 2 (activating) is initiated by binding of B7 molecules on the APC to CD28 receptors on the T cell
signal 3 (inhibitory) results from CTLA-4 expression on the T-cell surface, where it competes with CD28 for binding to B7 on APCs
CTLA-4 limits T-cell responses early in an immune response, primarily in lymphoid tissues
weak TCR signal
with a weak TCR signal, CD28:B7 binding predominates and a net positive activating signal, IL-2 production, cell proliferation, and enhanced survival
strong TCR signal
with a strong TCR signal, CTLA4:B7 binding predominates and results in a net negative signal, reduced IL-2 production, reduced cell proliferation and cell survival
Ipilimumab
CTLA-4 antibody — binding to CTLA-4 results in its neutralization and subsequent inhibition of effector T-cell inactivation
indicated for treatment of melanoma
well characterized family of melanoma associated antigens (MAGE) expressed in melanoma tissue and absent in normal tissues
ADEs and toxicity:
immune related adverse effects in the skin and GI due to immune enhancement
response to Ipilimumab can NOT be predicted based on objective biomarker level
immune checkpoint: PD-1
Programmed Death 1 (PD-1) expressed on T-cells and bind to Programmed Death Ligands (PDL-1 or PDL-2)
PD-1 pathway regulates previously activated T cells at the later stages of an immune response, primarily in peripheral tissues
PD-1/PDL-1/2 is a co-inhibitory signal that results in inhibition of T cell activation, proliferation, and reduces T-cell survival
PD-1 ligands can be inducibly expressed by non immune cells, including tumor cells
tumors that over-express PDL-1/2 may be able to evade the immune response by inhibiting T-cell function at the level of the tumor microenvironment
mechanisms of immune system evasion by tumors
ineffective presentation of tumor antigens to the immune system
recruitment of immunosuppressive cells
release of immunosuppressive factors
factors/enzymes directly or indirectly suppress immune response
T-cell checkpoint dysregulation
PD-1/PD-L1 pathway
prolonged TCR (T cell receptor) stimulation during an ongoing immune response can cause upregulated PD-1 expression of T-cells
release of proinflammatory cytokines by T-cells upregulates PD-L1 expression by tumor cells or through oncogenetic mutations
PD-1/PD-L1 binding results in reduced T-cell proliferation, reduced proinflammatory cytokine production and reduced survival
PD-1 inhibitors
Nivolumab (Opdivo)
Pembrolizumab (Keytruda)
PD-L1 inhibitors
Durvalumab (Imfinzi)
predictive marker for response to PD-1 and PDL-1 inhibitors
tumor PD-L1 expression can be used a s a predictive marker for response to the use of PD-1 and PD-L1 inhibitors
PD-L1 is used as a marker to determine patient eligibility to receive these treatments
immune related ADEs — CTLA-4 vs PD-1 and PD-L1
higher prevalence w/ CTLA-4 b/c it’s non-specific in stimulating immune response in lymph nodes at cancer cells and other normal cells
combined CTLA-4 and PD-1/PD-L1 treatment
CTLA-4 pathway blockade allows more activation of T-cells in the lymph nodes and inhibits T-reg function
PD-1 pathway blockade restores the activity of anti-tumor T-cells at tumor site
monoclonal antibodies against cancer cell surface markers
Rituximab (chimeric) and Ofatumumab (fully human)
binding to CD20 on surface of malignant B-cell results in:
apoptosis
antibody-dependent cell-mediated cytotoxicity (ADCC)
complement-dependent cytotoxicity
Rituximab: the R in R-CHOP for B cell Non-Hodgkin’s Lymphoma
combining immunotherapy and radiotherapy: antibody-radionuclide conjugates
90Y-Ibritumomab
Ibritumomab — mouse monoclonal antibody against CD20 (similar target of Rituximab)
Tiuxetan — a linker-chelator that is covalently attached to the Mab and provides a high affinity chelating site for 90Y
90Y (Yttrium-90) — pure β radiation/particle emitter with a half-life of 64 hrs
β rays can penetrate soft tissues up to 4 mm
β radiation is a class of ionizing radiation that damages DNA resulting in DNA strand breaks
adoptive cell transfer — engineered T-cells
Tisagenlecleucel — cell based therapy for diffuse large B cell lymphoma
extracellular domain — antigen-recognition (antibody to antigen)
intracellular domain — when antigen binds to the extracellular domain, signaling by intracellular domain results in T cell activation and cancer cell destruction
CAR-T (Chimeric Antigen Receptor)-T cells:
re-engineer patient’s own T-cells to recognize a specific cancer antigen
transduction of the engineered antigen receptor into the patient's T cell is achieved using a virus
patient’s T-cells now express a CAR against CD19
when cells are re-infused into the patient, cells are now programmed to recognize CD19 and destroy CD19 bearing cells
interleukin-2 (IL-2)
produced primarily by Th1 subset and induces activation and proliferation of cytotoxic T cells
Alesleukin (Proleukin)
recombinant human interleukin-2 (IL-2)
endogenously produced by NK and T-cells (T helper cells)
major physiologic role: promote activation and proliferation of T and NK cells in autocrine and paracrine manner
toxicity — capillary leak syndrome
increase in leakiness of small blood vessels
characterized by hypotension, tachycardia, swelling of arms, legs, and other parts of the body and pulmonary edema
interferons (IFNs)
type I: IFN𝜶, IFNβ
type II: IFNɣ
IFNs activate dendritic cells, natural killer cells, and cytotoxic T cells and suppresses the function of myeloid derived suppressor cells (MDSC)
side effects:
flu-like symptoms, fatigue, neuropsychiatric