Edexcel a level chemistry topic 17
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
carboxyl group
COOH
physical properties of carboxylic acids
- able to form intermolecular H bonds due to hydroxyl group within their carboxyl group
- increasing boiling points with increasing carbon chain length
- solubility in water decreases with increasing carbon chain length
hydrogen bonding in carboxylic acids
- the effect of H bonding decreases as the alkyl chain increases in length because it is more likely to interrupt the H bonding that the carboxylic acid groups are able to make
- ethnic acid is an example of where the molecules are small enough that they are able to form dimerisation through H bonding
boiling points in carboxylic acids
- as the length of the carbon chain increases, the London forces between the non- polar hydrocarbon chain increase
- therefore, boiling temperature increases with increasing molecular mass, even though the effect of H bonding decreases
- the carboxyl group has 3 polar bonds, resulting in strong intermolecular forces and H bonding
- therefore, carboxylic acids have a higher boiling temp. compared to other organic compounds with a similar mass
solubility of carboxylic acids in water
- the effect of H bonding decreasing with increasing chain length affects solubility
- acids with 1-4 carbons are very soluble
- this is due to the highly polar C=O and O-H bonds, resulting in H bonds forming with water molecules
- as non- polar hydrocarbon chain becomes longer, solubility decreases
ways of preparing carboxylic acids
- oxidation of aldehydes
- oxidation of primary alcohols
- hydrolysis of esters
- hydrolysis of amides
- hydrolysis of nitriles
- hydrolysis of acyl chlorides
oxidation of primary alcohols and aldehydes
REAGENT: excess acidified potassium dichromate (VI), Cr2O7 2-/ H+
CONDITIONS: heat strongly under reflux
PRODUCT: carboxylic acid refluxing; separated by distillation
reduction of carboxylic acids
REAGENT: lithium tetrahydridoaluminate (LiAlH4)
CONDITIONS: dry ether
PRODUCT: primary alcohol and water
acid hydrolysis of esters
REAGENT: conc. sulfuric acid with excess water
CONDITIONS: heat
PRODUCT: alcohol and carboxylic acid
alkali hydrolysis of esters
REAGENT: sodium/ potassium hydroxide solution
CONDITIONS: heat
PRODUCT: alcohol and carboxylate salt (which can be converted to carboxylic acid by reacting with HCl)
esterification
REAGENT: carboxylic acid and alcohol with conc. sulfuric acid catalyst
CONDITIONS: heat
PRODUCT: ester
use of conc. H2SO4 in esterification reactions
- esterification is a reversible reaction which occurs at a slow rate
- H2SO4 is used as a drying agent
- H2SO4 increases yield and rate of reaction by dehydrating the system, causing the equilibrium to shift to the right and favour the forwards reaction
acyl chloride chemical properties
- attacked at the positive carbon centre by nucleophiles, such as water, alcohols, ammonia, amines
- undergo nucleophilic addition- elimination reactions
- more reactive than carboxylic acids and acid anhydrides
- very reactive with water
hydrolysis of acyl chlorides
REAGENT: acyl chloride + distilled water
CONDITIONS: RTP
PRODUCT: carboxylic acid and HCl
observations of hydrolysis of acyl chlorides
1. white misty fumes (HCl); damp universal indicator paper shows that the fumes are acidic because; fumes of ammonia mixed with HCl(g) produce a white smoke of ammonium chloride
2. fizzing and white solid
3. heat
preparation of acyl chlorides (halogenation)
REAGENT: carboxylic acid and phosphorus (V) chloride (PCl5)
CONDITIONS: RTP in anhydrous conditions
PRODUCT: acyl chloride, POCl3(l), HCl(g) (acyl chloride separated by fractional distillation)
observations of preparation of acyl chlorides
- vigorous reaction
- toxic HCl(g) forms
acid hydrolysis of nitriles
REAGENT: dilute HCl catalyst
CONDITIONS: heat under reflux
PRODUCT: carboxylic acid (separated by fractional distillation) and ammonium chloride
alkali hydrolysis of nitriles
REAGENT: sodium hydroxide
CONDITIONS: heat under reflux
PRODUCT: sodium carboxylate salt (reacts with HCl to produce carboxylic acid) and water
hydrolysis of aromatic nitriles
REAGENT: H2SO4 catalyst
CONDITIONS: heat under reflux
PRODUCT: carboxylic acid and ammonium sulphate
acyl chloride functional group
R-COCl
acyl chlorides and water
- react violently; reactions using acyl chlorides should be one in anhydrous conditions
- produces carboxylic acid and HCl
acyl chlorides and alcohols
- react readily
- form an ester and HCl gas
amines
- homologous series that contain the R-NH2 group
- react similarly with most carboxylic acid derivatives
- able to form N-substituted amides
ethanoyl chloride and methylamine (primary amine)
forms N-methylethanamide (secondary amide) and HCl
ethanoyl chloride and dimethylamine (secondary amine)
forms N, N-dimethylethanamide (tertiary amide)
ethanol chloride and tertiary amines
no reaction occurs
acyl chlorides and conc. ammonia
- forms an amide and HCl(g)
- a further reaction occurs, where basic NH3 reacts with acidic HCl to form NH4Cl
carbonyls
aldehydes and ketones
- contains C=O group
aldehyde group
RCHO
ketone group
RCOR'
formation of aldehyde
REAGENT: primary alcohol, potassium dichromate (VI)
CONDITIONS: heat gently and distill
PRODUCT: aldehyde
formation of ketone
REAGENT: secondary alcohol, potassium dichromate (VI)
CONDITIONS: heat under reflux
PRODUCT: ketone
prefix for ketone when it doesn't take priority
oxo-
physical properties of carbonyls
- C=O bond has highly electronegative O atom
- cannot intermolecular form H bonds due to lack of -OH group
- strongest intermolecular force that can be made is dipole- dipole forces
- only the smallest carbonyl compounds can form H bonds with water; this is due to the lone pair on the oxygen in the carbonyl group, which bonds with +ve H atoms in H2O molecules
- solubility decreases as carbonyl chain length increases
- BP increases with increasing chain length
why does solubility decrease as carbonyl size increases?
- longer alkyl chains disrupt the carbonyl compound from forming H bonds effectively
- regions of molecules are hydrophobic
order of BP of some organic molecules from highest to lowest
1. alkanes
2. ketones
3. aldehydes
4. alcohols
BP of ketones
- polar carbonyl group means that both London forces and dipole- dipole interactions are present
bond angle around a carbonyl group
120 degrees
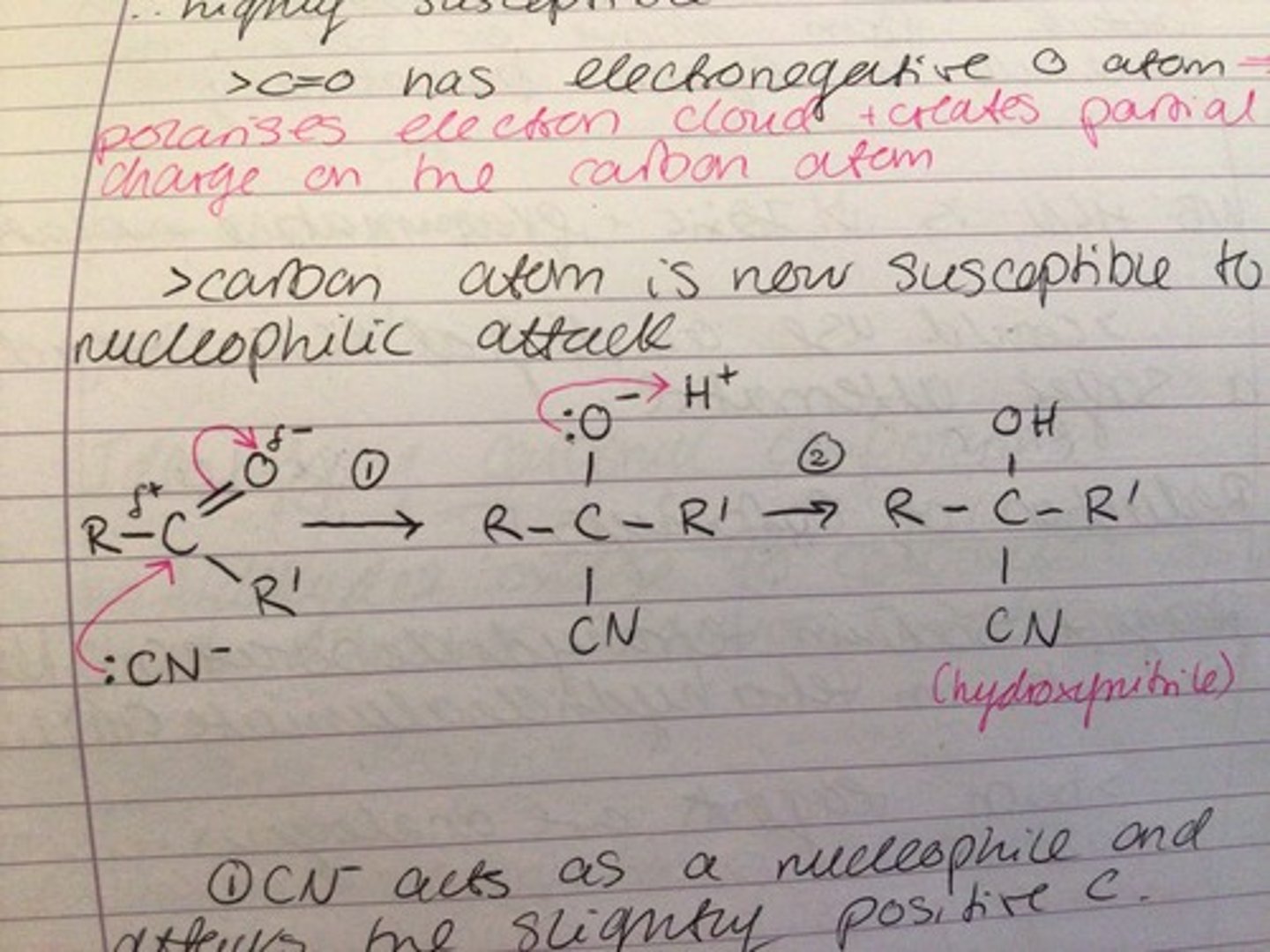
Why are carbonyls susceptible to nucleophilic attack?
- carbonyls are highly susceptible to attack because of the pi bond electron clouds above and below the C=O bond
- the oxygen in the C=O bond is electronegative, so polarises the electron cloud and creates a partial charge on the carbon atom, therefore the carbon atom is now susceptible to electrophilic attack
Nucleophilic addition of carbons mechanism (HCN or KCN)
1. CN- acts as a nucleophile and attacks the slightly positive carbon
2. One of the C=O bonds breaks; a pair of electrons goes to the oxygen atom, which will now have a negative charge
3. A pair of electrons from the oxygen atom is used to form a bond with H+
4. Overall addition of CN to the molecule, therefore increasing carbon chain length
5. Hydroxynitrile produced can be optically active, but does not rotate plane polarised light
6. This is because the nucleophile can attack from above or below the molecule with equal probability to form a racemate
Why should acidified KCN be used instead of HCN in nucleophilic addition?
HCN is v. toxic and flammable and volatile
KCN is safer alternative
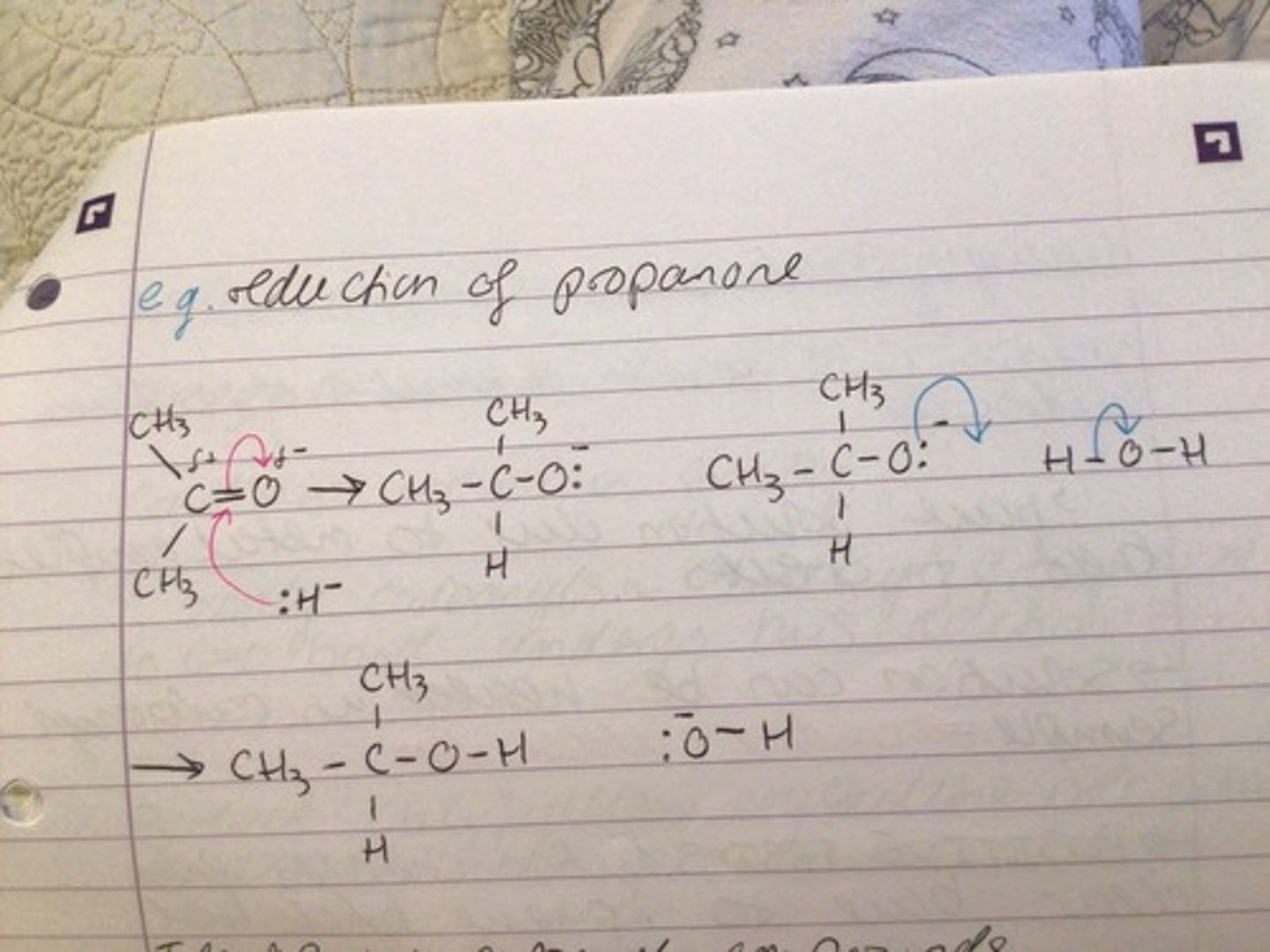
Reduction of carbonyls
REAGENT: sodium tetrahidridoborate (III) NaBH4 or Lithium tetrahydridoaluminate LiAlH4
CONDITIONS: dry ether
NUCLEOPHILE: H- (hydride ion)
MECHANISM: nucleophilic additions
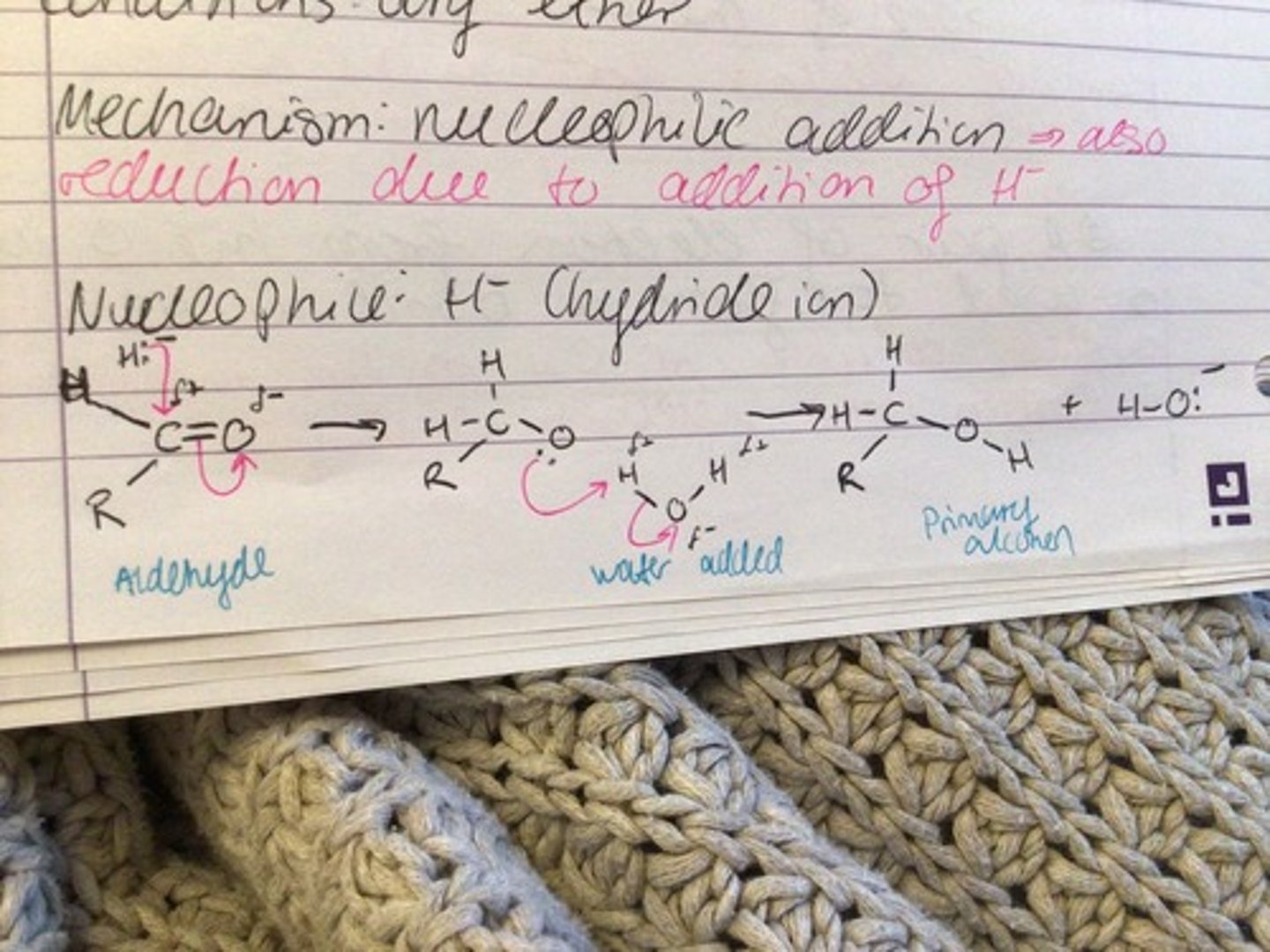
Reduction of propanone
Oxidation of carbonyls
Aldehydes can oxidise to carboxylic acids but ketones cannot further oxidise
Tollens reagent
[Ag(NH3)2]+
- acts as an oxidising agent when heated gently with a carbonyl containing solution
Positive test for tollens
Silver mirror produced when aldehyde is present
Fehlings solution
- contains Cu2+ ions in a solution of sodium hydroxide
- solution oxidises aldehydes when heated with a sample
Positive Fehling's test
Solution changes from clear blue to opaque brick (due to copper(I) oxide being formed) in presence of aldehydes
Positive test for potassium dichromate
Orange to green in aldehydes
Why are carbonyl compounds difficult to distinguish?
They all have similar boiling points
Iodine in presence of alkali
REAGENTS: iodine + NaOH
CONDITIONS: warm very gently
PRODUCT: CHI3, a yellow crystalline precipitate with a distinct antiseptic smell
- only carbonyls with a methyl group next to the C=O bond undergo this, so mainly ketones and ethanal
2,4-dinitrophenylhydrazine
- C=O undergoes condensation reaction under acidic conditions with 2,4-DNPH
- when carbonyl is present, deep orange precipitate forms, which is purified by recrystallisation
- derivatives formed post- reaction gives clear and siting melting and boiling points
Structural isomerism
Same molecular formula, different structural formula
Chain isomerism
- isomers with different arrangement of carbon skeleton
- similar chemical properties
- slightly different physical properties
- more branches= lower BP
Position isomerism
-isomers with same carbon skeleton and same functional group
- functional group is in a different position
- similar chemical properties and slightly different physical properties
Functional group isomerism
- isomers with different functional groups
- exhibit different chemical and physical properties
Stereoisomerism
-Same molecular formula but atoms occupy different positions in space
- relies on existence of at least 2 groups
- on two different carbons
- bonded together by a C=C double bond, which restricts rotational movement
Geometrical isomerism
- occurs due to the restricted rotation of the C=C double bonds
- E-Z isomerism
- cis- trans isomerism
Optical isomerism
- Occurs when molecules have a chiral centre
- 2 non- superimposable mirror images
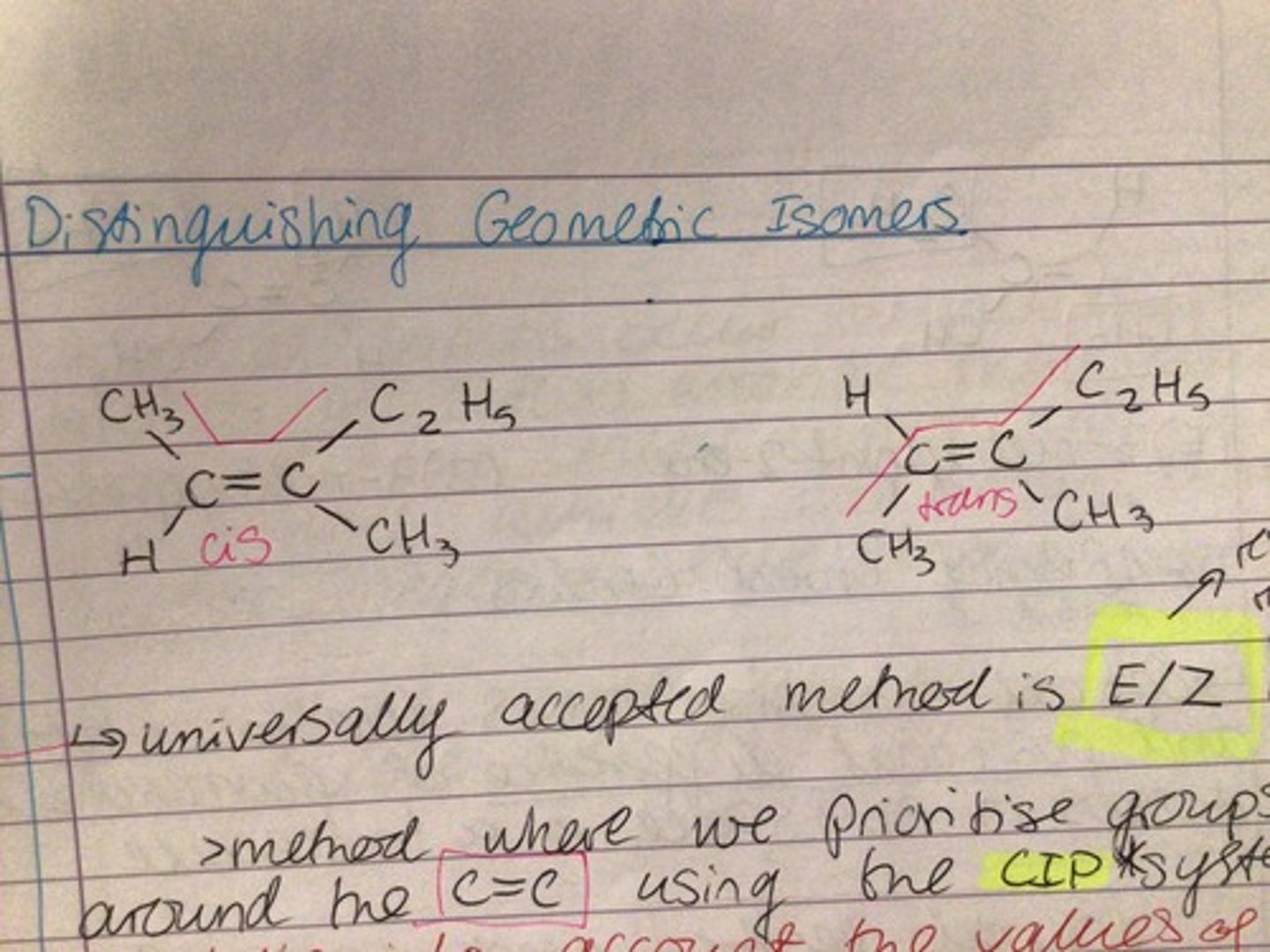
E/Z isomerism
- groups around C=C bond are prioritised by their atomic numbers
- E( entgegen): higher priority group on opposite sides of C=C bond
- Z( zusammen): higher priority group on same side of C=C bond
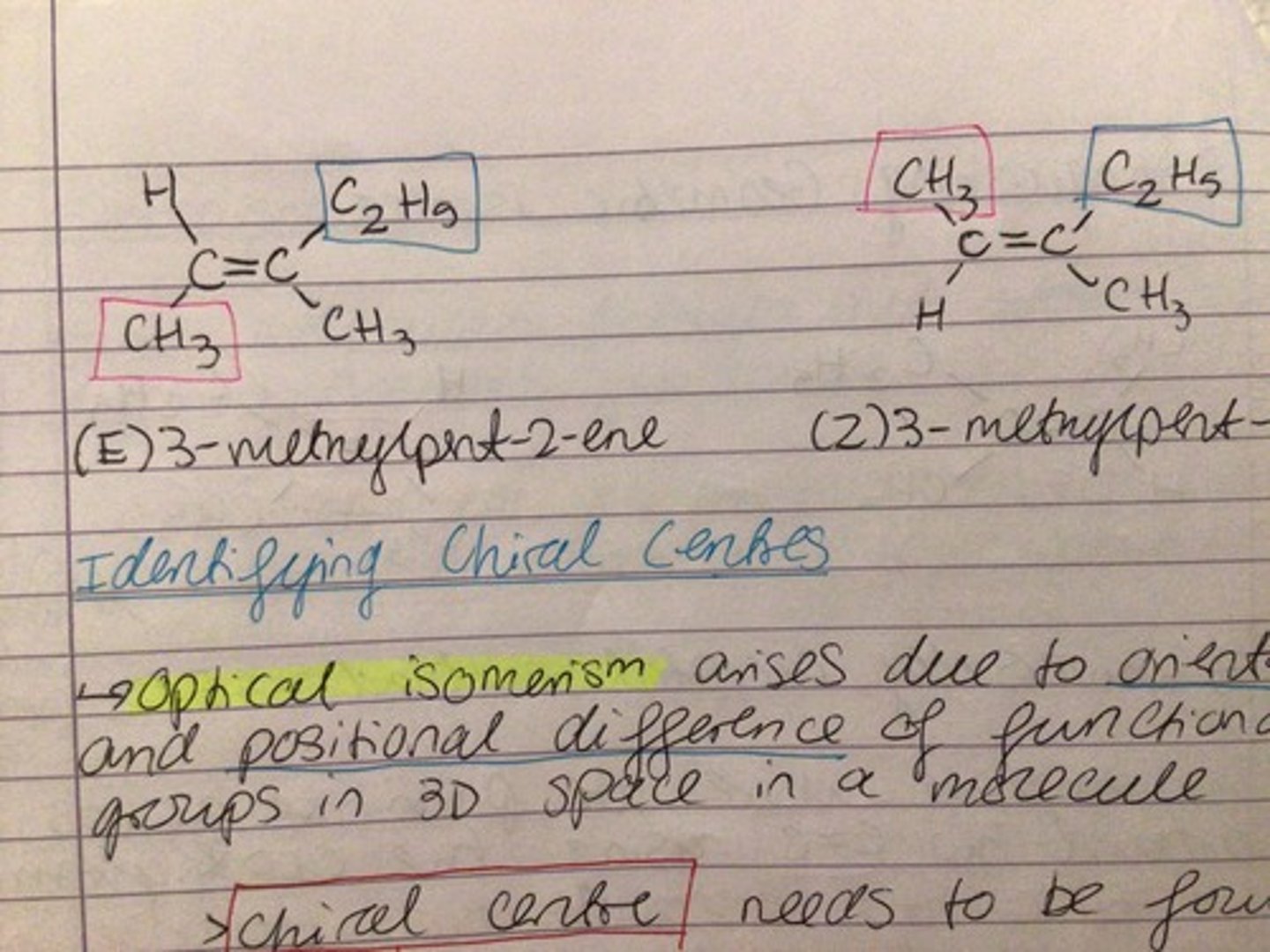
Cis-trans isomerism
- based on longest carbon chain present
Identifying chiral centres
- optical isomerism arises due to orientation and positional difference of functional groups in 3D space in a molecule
- chiral centre causes this
Chiral centre
Carbon atoms that are found in the molecule that have different/ unique groups of molecules bonded to it, arranged in a tetrahedral structure
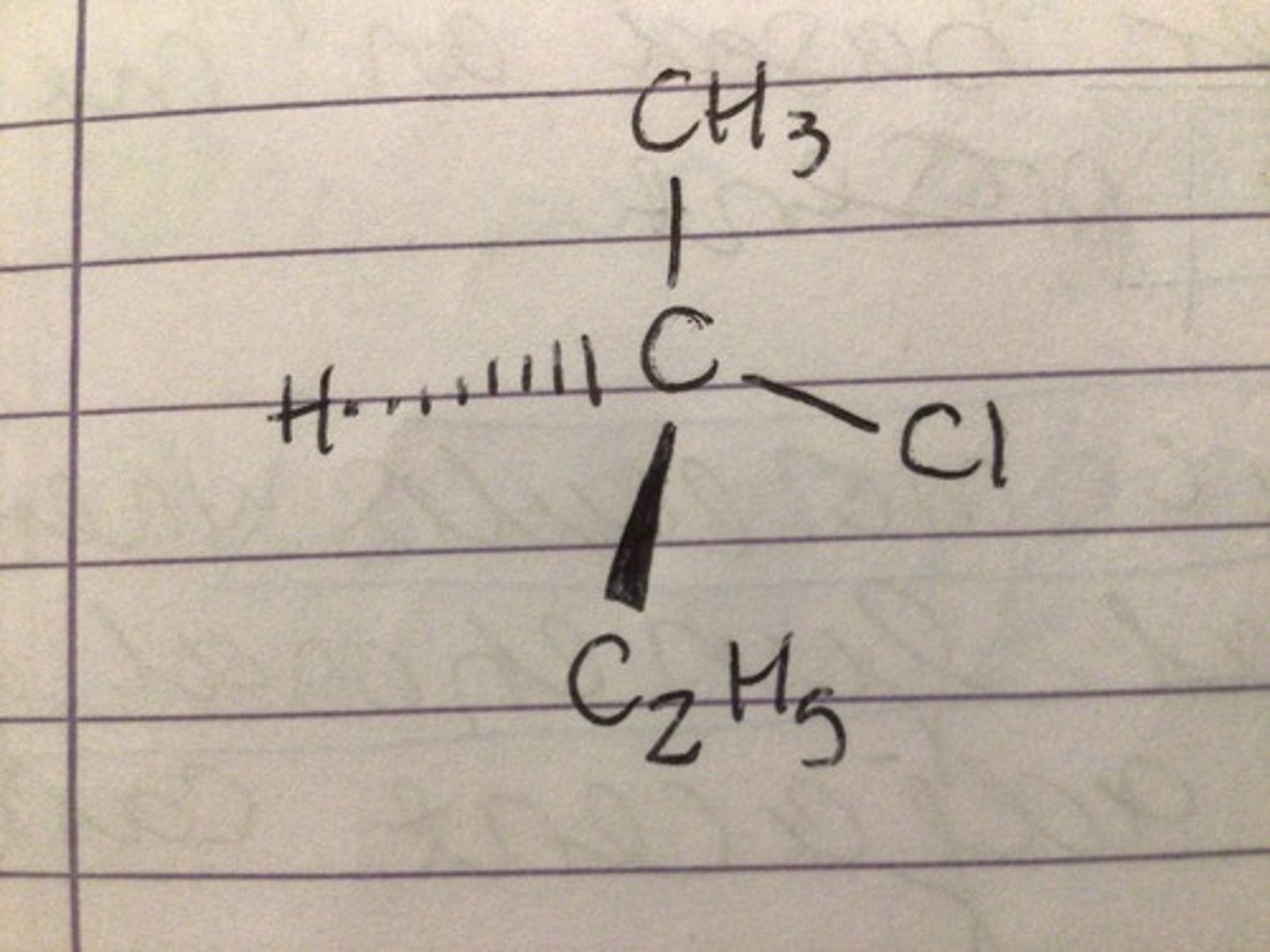
Enantiomers/ conditions for optical isomerism
- mirror images of each other
- non- superimposable
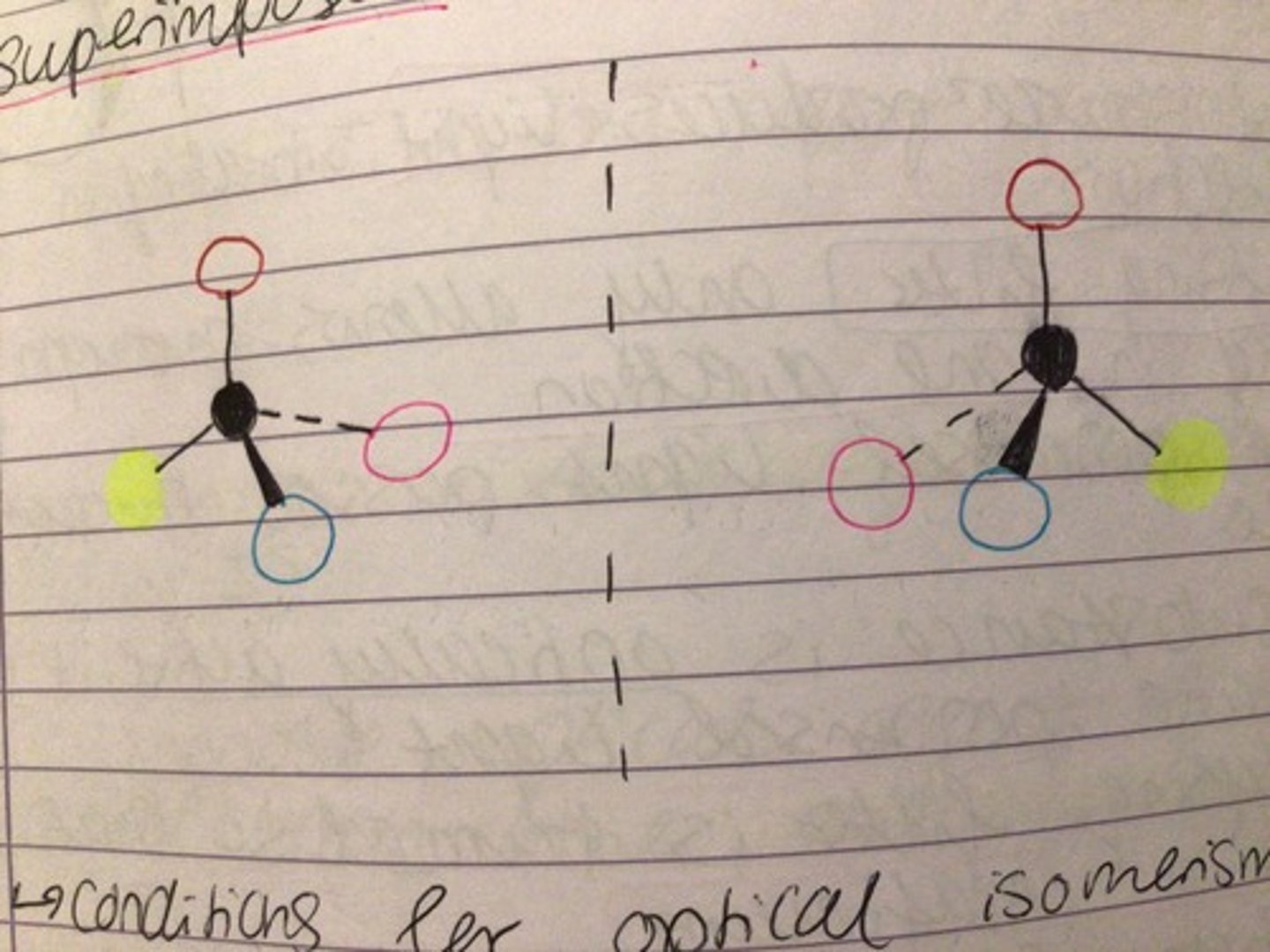
How to distinguish enantiomers
- they should rotate plane polarised light in two different directions
Polorimeter
1. Light source produces light vibrating in all directions
2. Polarising filter only allows through light vibrating in one direction
3. Plane polarised light passes through samples
4. If sample is optically active, it rotates the plane polarised light
5. Analysing filter is turned so that light reaches a maximum
6. Direction of rotation is measured coming towards the observer
Dextrorotatory
Rotates plane-polarized light to the right
Laevorotatory
rotates plane polarised light to the left
Racemate
- 50/ 50 mixture of the two enantiomers
- opposite optical effects of each isomer cancel each other out
Formation of enantiomers
- carbonyl compounds undergo nucleophilic addition
- if there are two different groups attached to the C=O bond, the possibility of forming optical isomers arises
E.g. is CN- attacks carbonyl from above, one optical isomer is formed, and another is formed if it attacks from below the planar molecule
- two non- superimposable mirror images produced
- race mix mixture because there is a 50/ 50 chance of attack from either above or below the plane
Thalidomide
- used to treat anxiety/ morning sickness is pregnant women
- many babies born with deformities/ missing limbs
- only one enantiomer was safe
racemic mixture
50/50 composition of each enantiomer
- there would be no rotation of plane polarised light because they cancel each other out