Ch 6: Metabolism
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
where does the energy that sustains most of the earth’s life forms comes from?
the sun
bioenergetics
study of energy flow through a living system
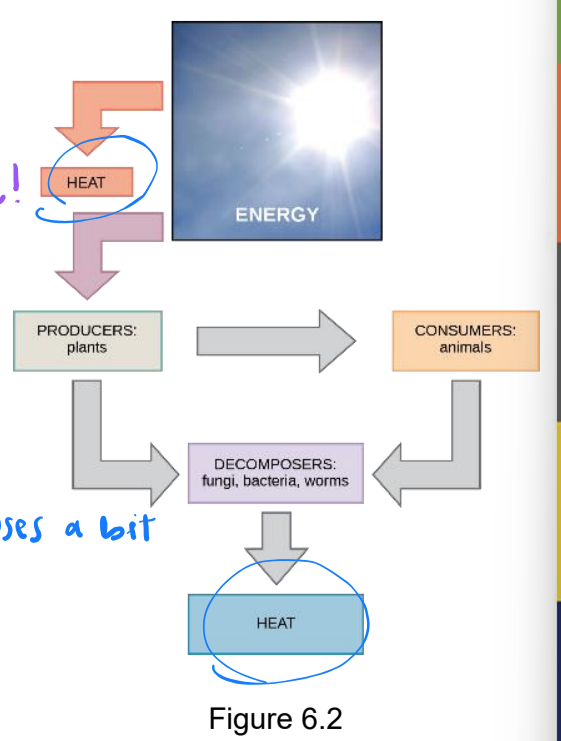
metabolism
all chemical reactions of a cell or organism
metabolic pathway
series of biochemical reactions that converts one or more substrates into a final
2 types of reactions/pathways required to maintain cell’s energy balance
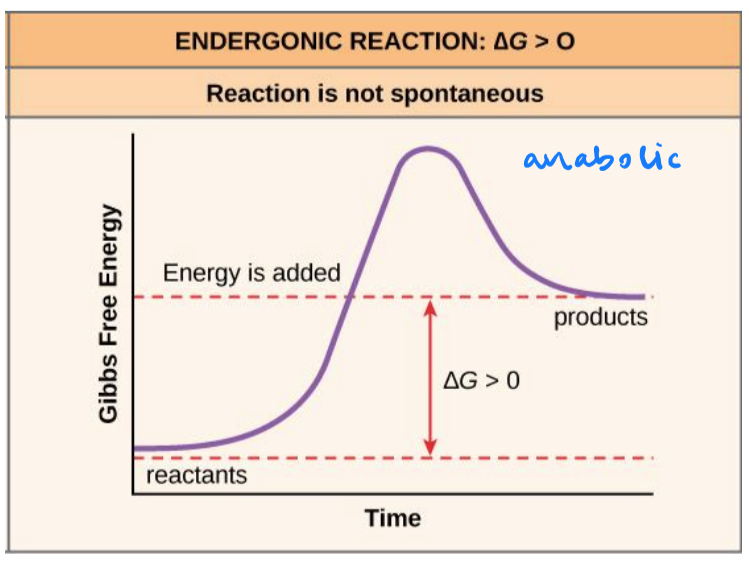
anabolic
catabolic
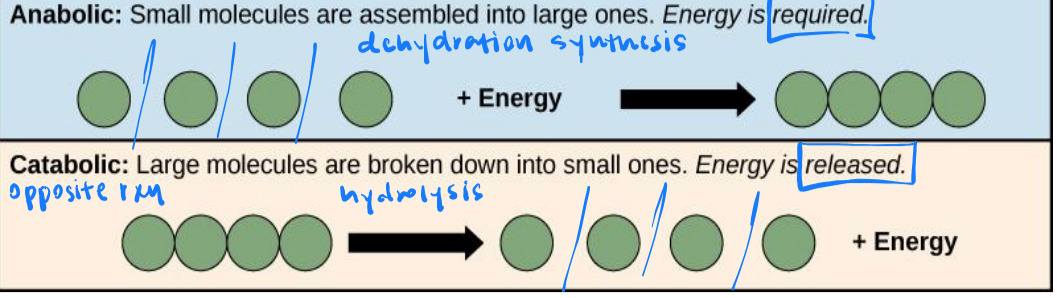
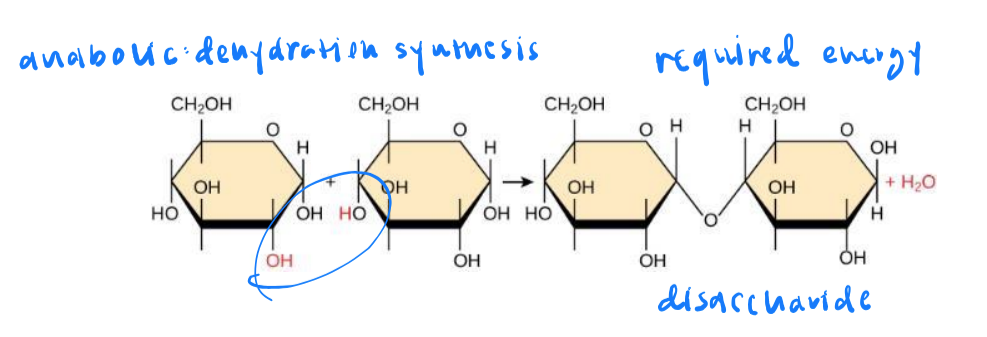
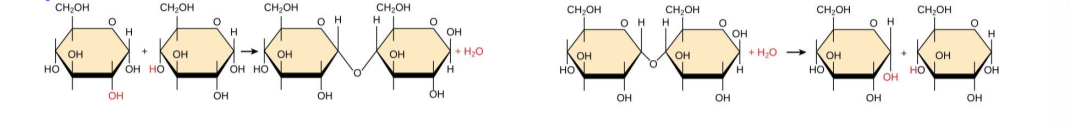
anabolic metabolic pathways
small molecules are assembled into large ones. energy is required.
catabolic metabolic pathway
large molecules are broken down into small ones. energy is released
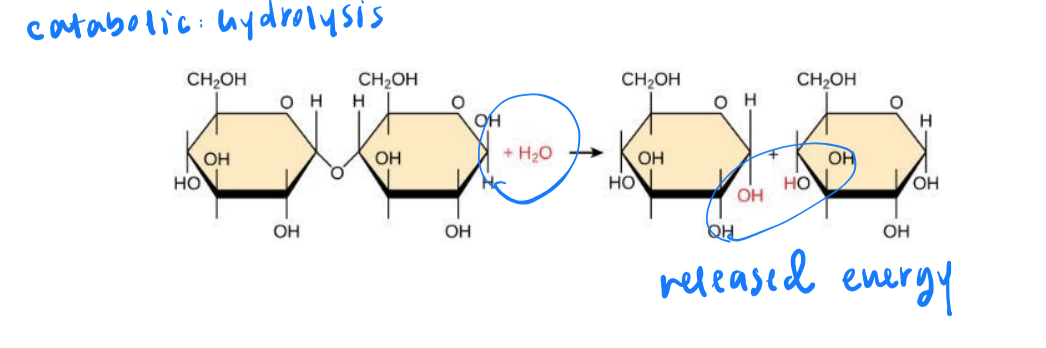
energy
ability to do work
objects in motion have kinetic energy
objects that have the potential to move have potential energy
chemical energy
energy stored in chemical bonds (potential) then released (kinetic)
bioenergetics of a system
the amount of energy exchanged in metabolic reactions
gibb’s free energy (G)
the amount of energy available to do work (usable energy)
all chemical reactions affect G
ΔG = change in G after a reaction
ΔG = ΔH-TΔS
ΔH = change in total energy of the system (enthalpy)
T is the temperature in Kelvins (oC + 273)
ΔS is change in entropy (energy lost to disorder)
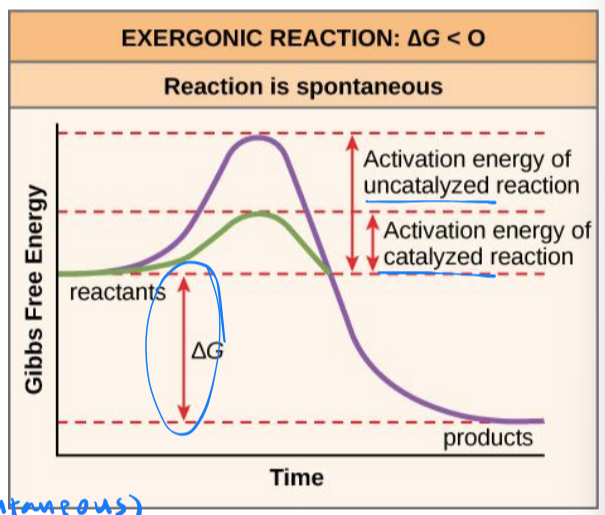
exergonic reactions
energy is released in a chemical reaction
ΔG < 0
products will have less free energy than substrates
are spontaneous because they can occur without addition of energy (reactants are enough)
spontaneous reactions do not necessarily occur quickly
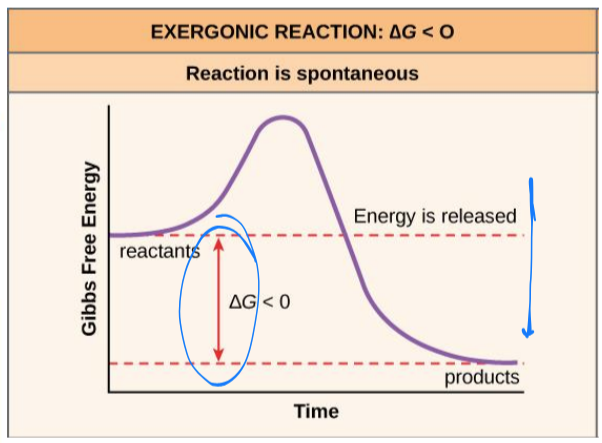
endergonic reactions
chemical reaction required an input of energy
ΔG > 0
products will have more free energy than substrates
not spontaneous/ will not occur quickly
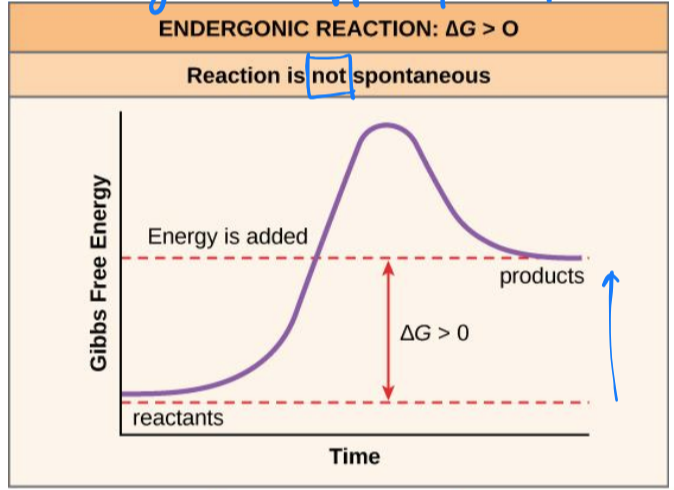
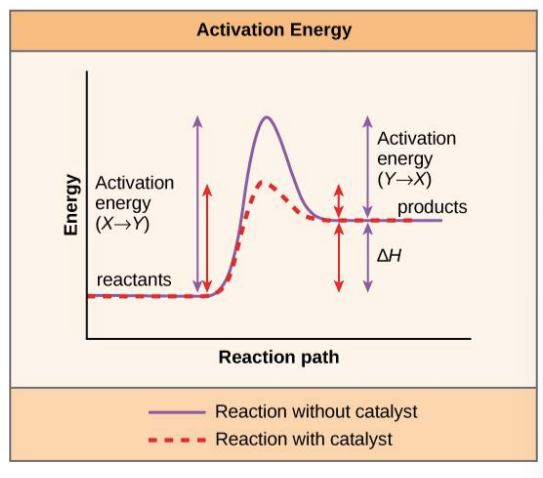
activation energy
the energy required for a reaction to proceed
causes reactant(s) to become contorted and unstable, allowing bond(s) to be broken or made
unstable state is called the transition state
in transition state the reaction occurs very quickly
what is the main source for activation energy in a cell?
heat energy
helps reactants reach transition state
thermodynamics
study of energy and energy involving physical matter
first law of thermodynamics
the total amount of energy in the universe is constant
energy cannot be created or destroyed
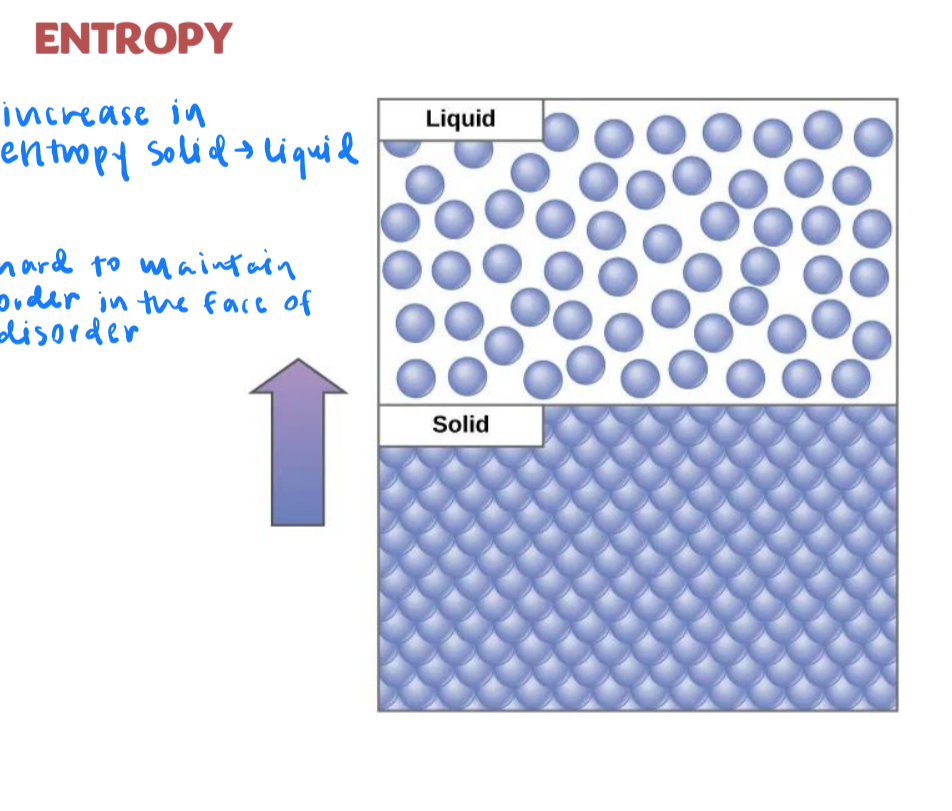
second law of thermodynamics
the transfer of energy is not completely efficient
in chemical reactions some energy is lost and unusable (i.e. heat energy)
increases entropy (disorder)
cell has to work harder to keep order: hard to maintain order in the face of disorder
ATP: adenosine triphosphate
provides the energy for a cell’s endergonic reactions
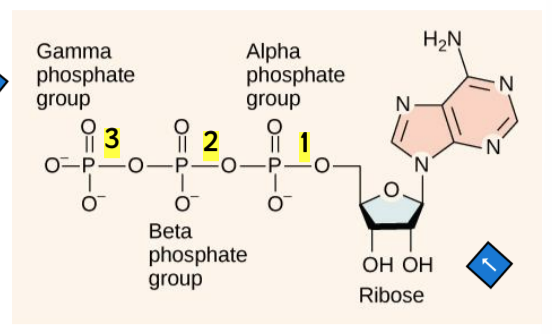
ATP structure
composed of adenosine backbone with 3 phosphate groups attached
adenosine = nitrogenous base adenine + 5-carbon ribose
3 phosphate groups: alpha, beta, and gamma
more phosphates → more unstable → easier to break
bonds between phosphate groups are high-energy
when broken the products have lower free energy than reactants
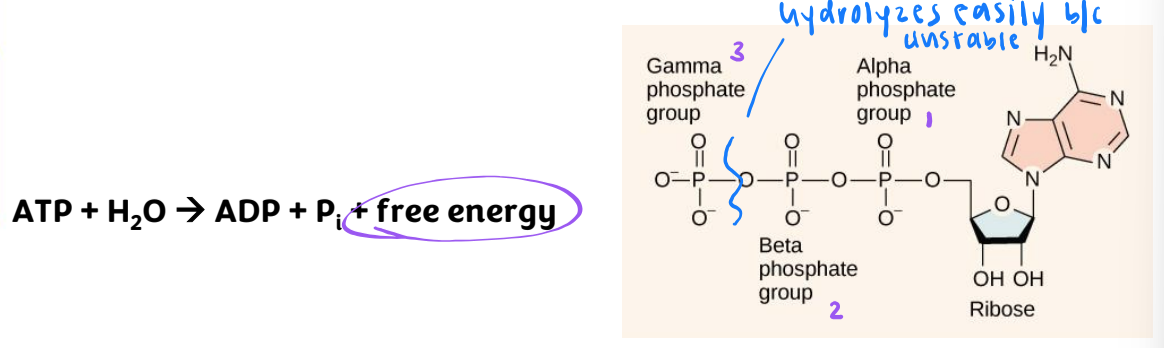
ATP hydrolysis
ΔG = -7.3 kcal/mol (nearly double in cells)
ATP is unstable and hydrolyzes quickly
energy lost as heat if not coupled to endergonic reaction
when coupled with an endergonic reaction much of the energy can be transferred to drive that reaction
ATP hydrolysis is reversible
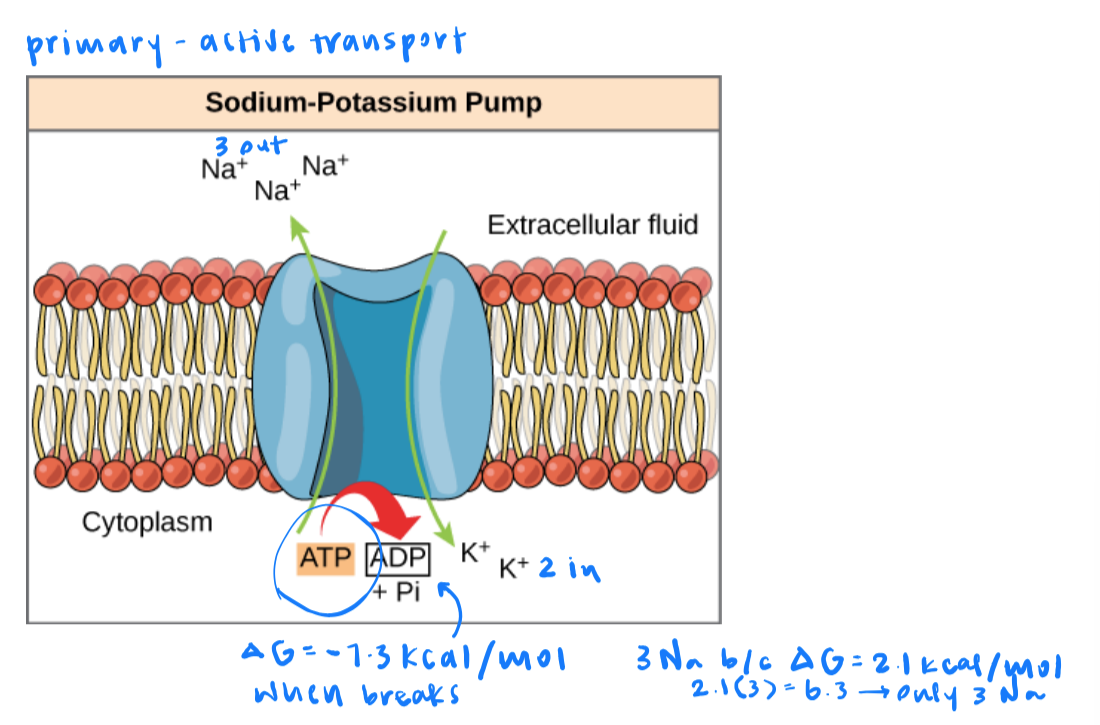
the sodium-potassium pump
enzymes
primarily protein catalysts that speed up reactions by lowering the required activation energy
bind with reactants and promote bond-breaking and bond-forming processes
very specific, catalyzing a single reactions
do not change reaction’s ΔG
enzymes are specific to actions → big metabolic forces need a lot of enzymes
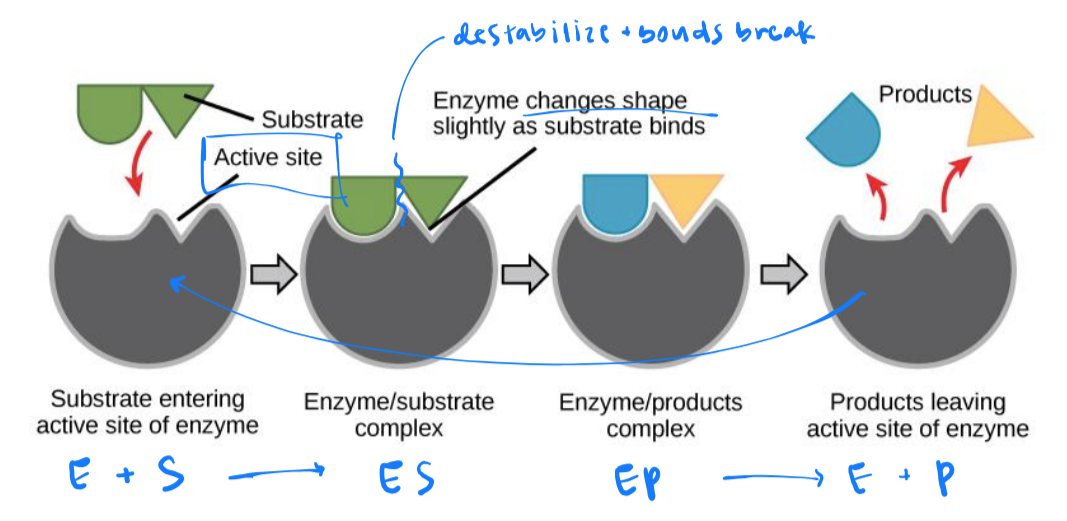
enzyme-substrate specificity
3d shapes (enzyme and substrates) determine specificity
substrates interact at enzyme’s active site
enzymes can catalyze a variety of reactions
3d image of enzyme active site
protein structure: scaffold to support and position active site
active site
binding sites: bind and orient substrate(s)
catalytic site: reduce chemical activation energy
enzyme induced fit
at active site, a mild shift in shape optimizes reactions
slight changes maximize catalysis
enzyme remains unchanged following reaction (resets)
protein structure revisited
3d shape of protein determined by amino acid sequence
AAs of active site important for enzyme’s function - allow binding with unique substrates
cellular environment important enzyme function
what are important considerations for the cellular environment for enzyme function?
suboptimal temperatures can denature the enzyme (loss of shape)
suboptimal pHs can reduce substrate-enzyme binding
lower temp slows down reactions b/c enzymes are moving slower
increased temp denatures enzymes to tertiary or secondary structure
fever: temp is raised just enough to speed up the immune system
lowering activation energy
an enzyme can help the substrate reach its transition state in one of the following ways
position two substrates so they align perfectly for the reaction
provide an optimal environment (i.e. acidic, polar) within the active site for the reaction
contort/stress the substrate by destabilizing the bonds so it is less stable and more likely to react
temporarily react with the substrate (chemically change it) making the substrate less stable and more likely to react
enzyme regulation
helps cells control environment to meet their specific needs
how can enzymes be regulated?
modifications to temperature and/or pH
production of molecules that inhibit or promote enzyme function
availability of coenzymes or cofactors
regulate expression at DNA level (transcription/translation)
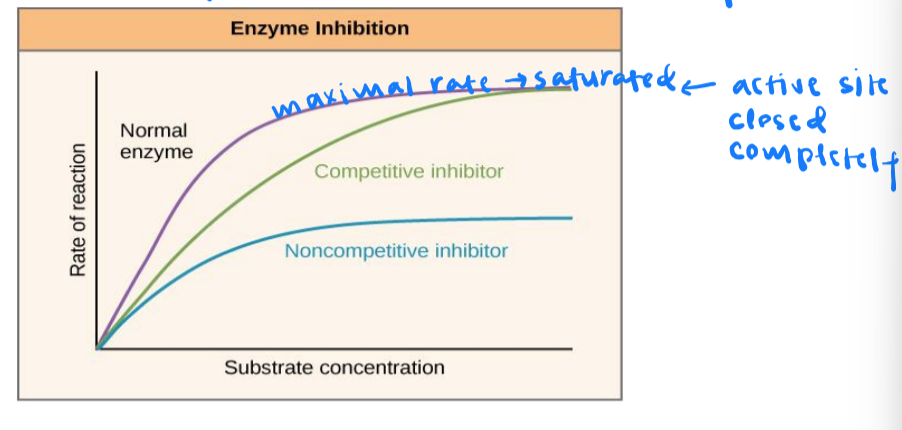
what are the 2 ways to inhibit enzymes?
through competitive inhibitors and noncompetitive inhibitors
competitive inhiibitors
have similar shape to substrate and compete w/ substrate for active site
noncompetitive inhibitors
bind to enzyme at different location (allosteric site) and slows reaction rate
enzyme inhibition
competitive inhibition slows reaction rates but does not affect the maximal rate
noncompetitive inhibition slows rates and reduces the maximal rate
maximal rate: speed of a reaction when substrate is not limited → saturation: all active sites are taken up
allosteric inhibitors
modify active site = substrate binding is reduced or prevented
allosteric activators
modify active site = affinity for substrate increases
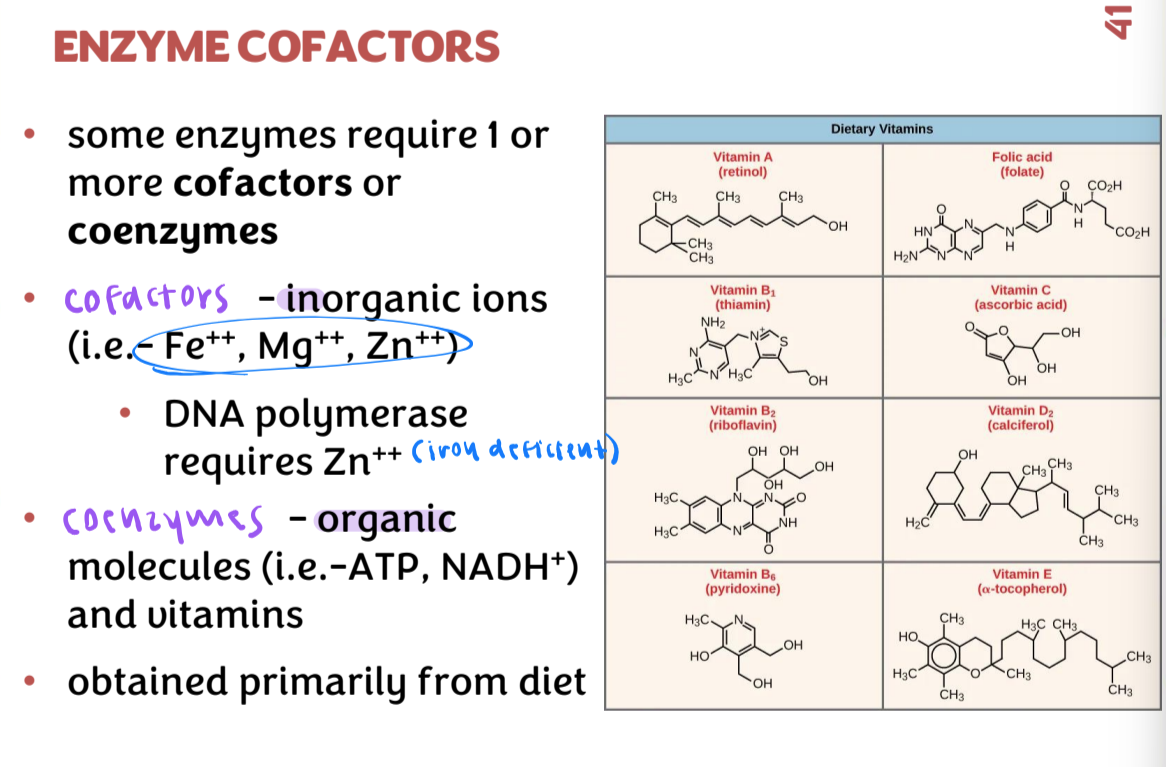
enzyme cofactors
some enzymes require 1 or more cofactors or coenzymes
cofactors: inorganic ions
coenzymes: organic molecules and vitamins
obtained primarily from diet
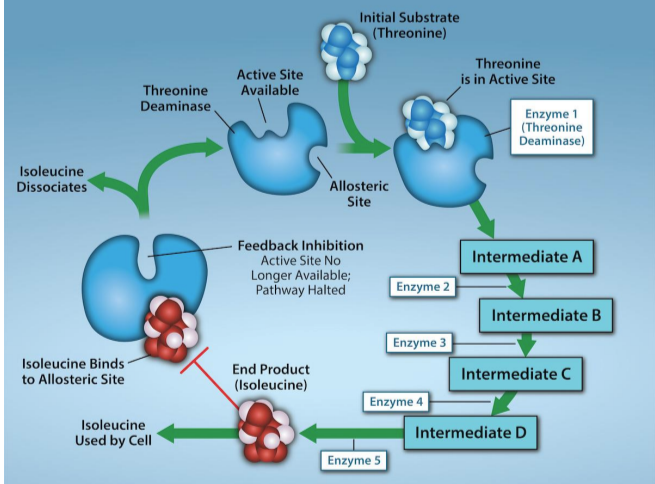
feedback inhibition in metabolic pathways
end-product of pathway inhibits an upstream step
important regulatory mechanism in cells
ex. ATP allosterically inhibits some enzymes involved in cellular respiration