Effects on Enzyme Activity
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Initial effect of increasing temperature
As the temperature increases, the enzyme and the substrate gain kinetic energy.
There are more frequent and successful collisions between the substrate and the active site.
There are more enzyme-substrate complexes forming.
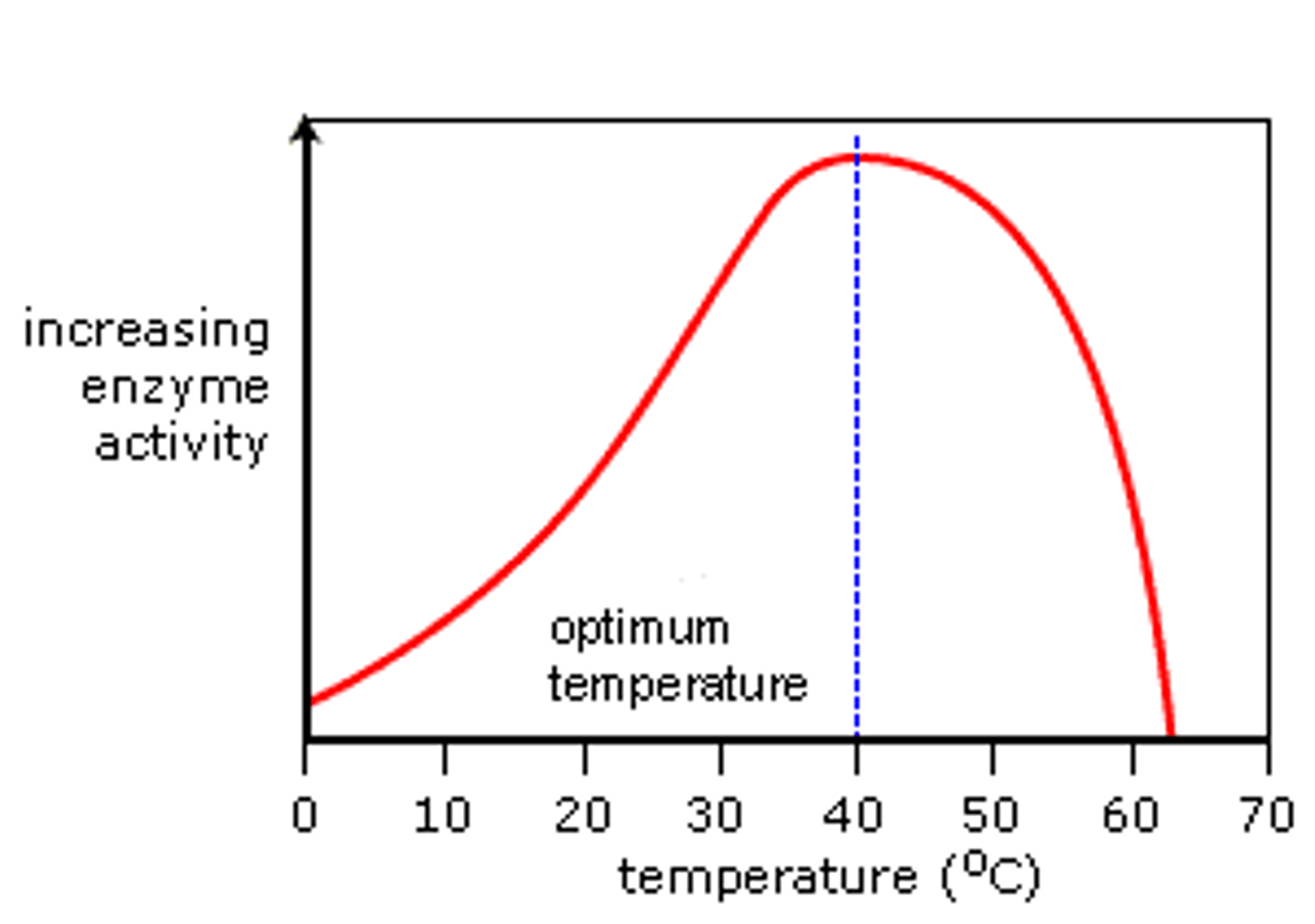
After optimum temperature
After the optimum temperature, due to gaining a lot of kinetic energy, the molecules in the enzyme move around more. This breaks hydrogen and ionic bonds, denaturing the enzyme.
The active site is no longer complementary or specific in shape to the substrate. The tertiary structure of the enzyme changes, and thus fewer enzyme-substrate complexes are formed.
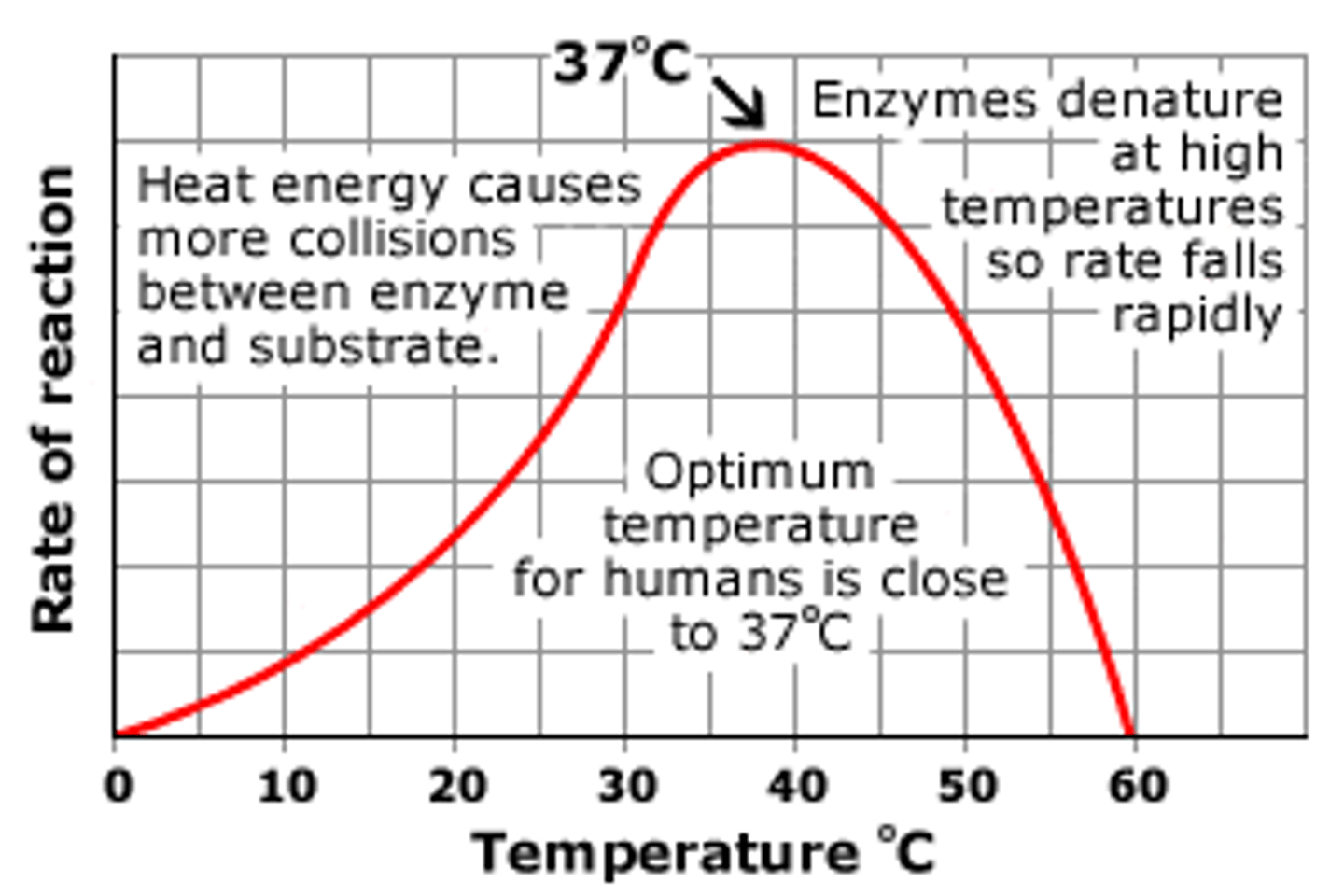
Temperature Coefficient
Q₁₀ is the temperature coefficient. it represents how much the rate of reaction changes when the temperature is increased by 10°C

The rate of reaction is 10 products produced per minute for a reaction occurring at 30°C.
The rate of reaction is 5 products produced per minute for the same reaction occurring at 20°C.
Q₁₀ for that reaction would be:
10/5 = 2
Q₁₀ = 2 tells us that the rate of reaction doubles when the temperature of the reaction is increased by 10°C.
A Q₁₀ = 3 would indicate that the reaction triples with a 10°C increase, and a Q₁₀ = 4 indicates the reaction quadruples.
Changes in pH
Denaturation can occur at low or high pH. The enzyme is affected due to disruption of the ionic and hydrogen bonds in the tertiary structure, which leads to an alteration in the specific shape of the active site.
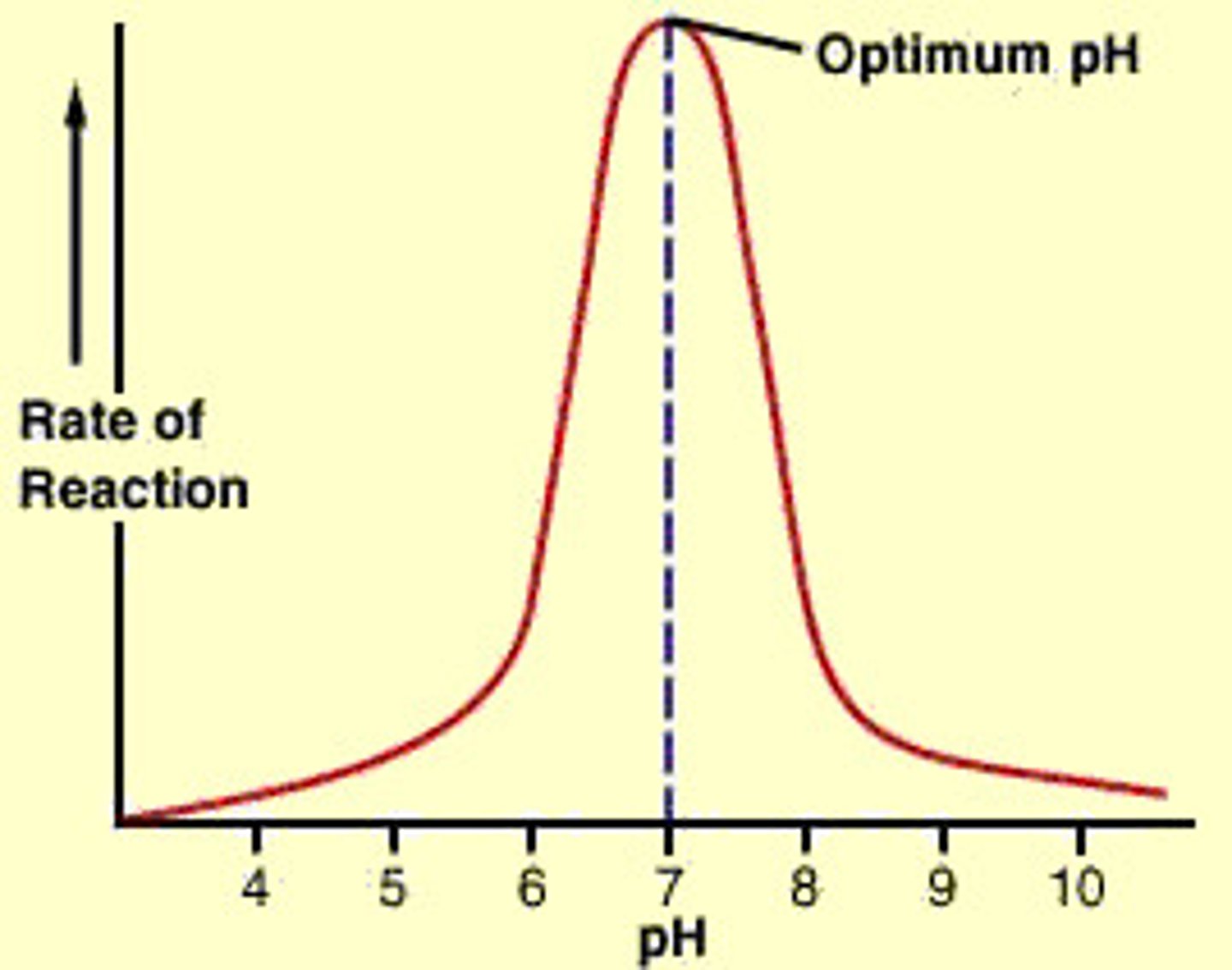
Effects of a low pH
At low pH (more acidic), the H⁺ attracts the negative R groups in the hydrogen and ionic bonds.
This causes the hydrogen and ionic bonds to break.
This changes the tertiary structure of the enzyme.
The active site is no longer complementary or specific in shape to the substrate. There are fewer enzyme-substrate complexes formed.
Effects of a high pH
At a high pH (mre alkaline), the OH⁻ attracts the positive R groups in the hydrogen and ionic bonds, causing the hydrogen and ionic bonds to break.
This changes the tertiary structure of the enzyme.
The active site is no longer complementary or specific in shape to the substrate. There are fewer enzyme-substrate complexes formed.
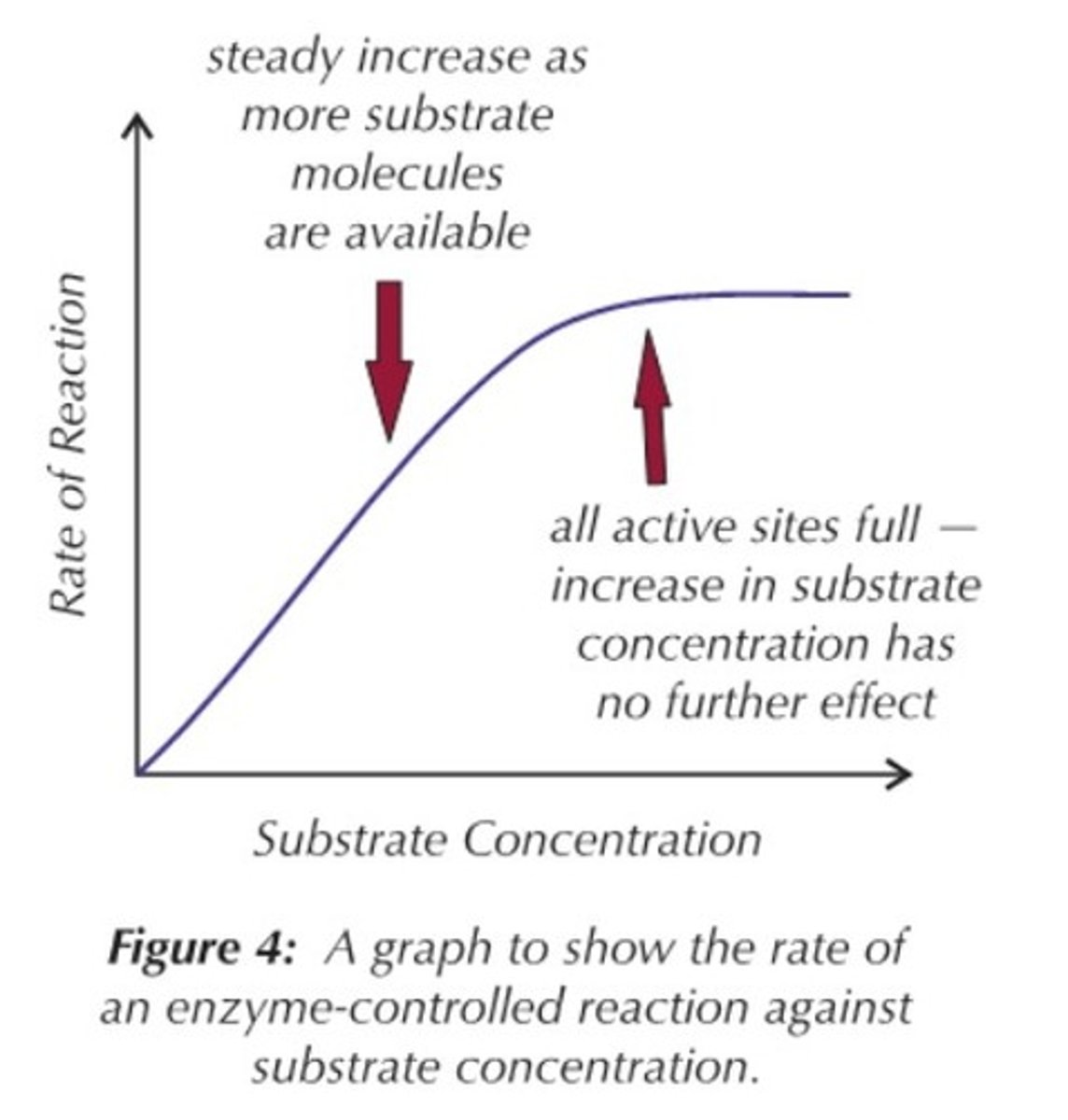
Effects of Substrate Concentration
As the substrate concentration increases, there are more successful collisions between the active site and the substrate.
There are more enzyme-substrate complexes formed, until all active sites are saturated and the enzyme concentration becomes the limiting factor.
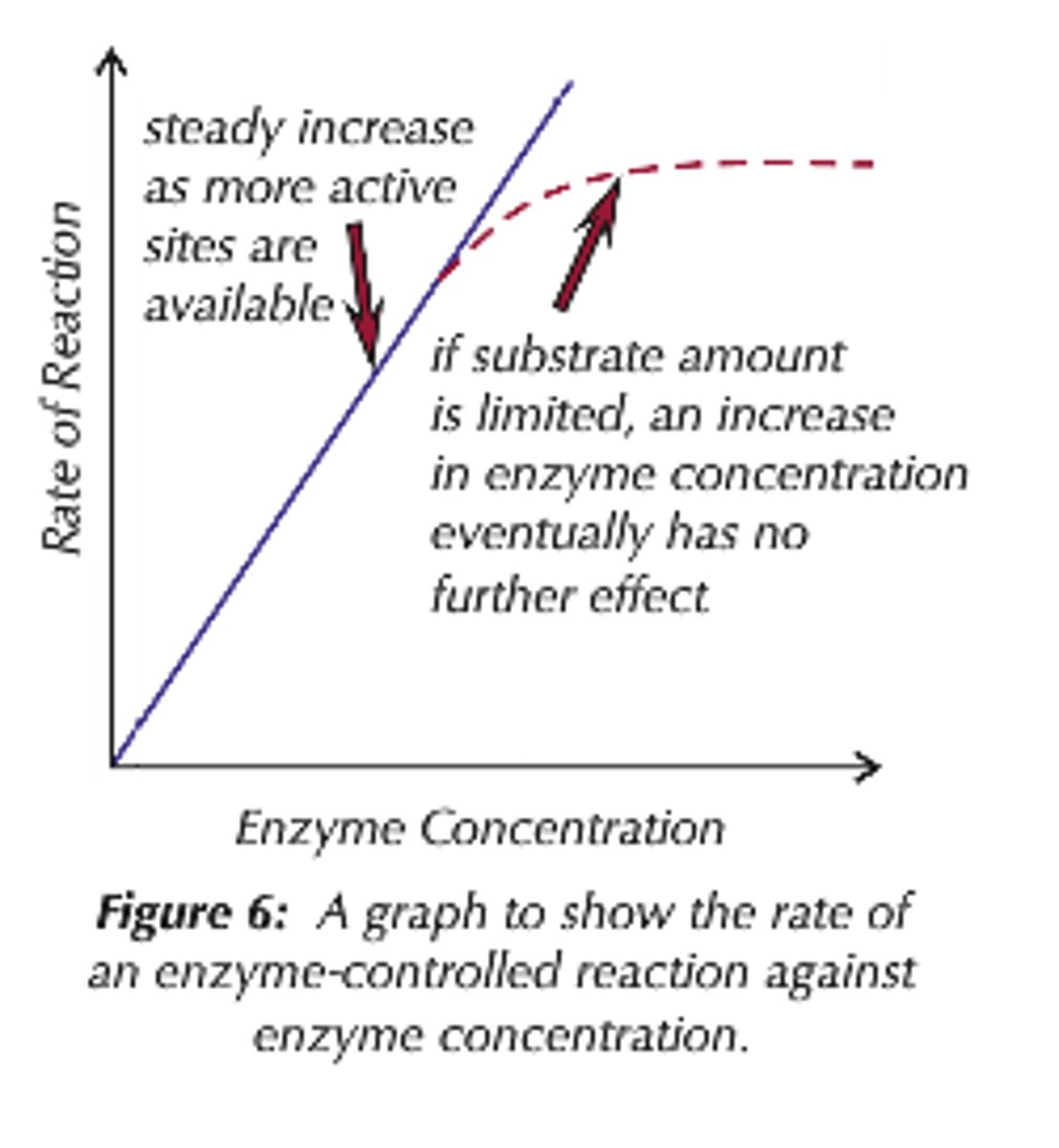
Effects of Enzyme Concentration on Rate of Reaction
As the enzyme concentration increases, there are more successful collisions between the active site and the substrate.
There are more enzyme-substrate complexes formed, until all the substrate is used up and the substrate concentration becomes the limiting factor.
Control Variables when investigating the effect on Enzyme Activity
Keep the variable you AREN'T testing the same.
pH, temperature, enzyme concentration. substrate concentration
CSMR (exam tip)
C - What you Change
S - What you keep the Same
M - What you are Measuring
R - Repeats