OCHEM 2 Textbook Flashcards
1/438
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
439 Terms
Infrared Spectroscopy
observes the vibrations of bonds and provides evidence of functional groups present
mass spectrometry
is not a spectroscopic technique, because it does not measure absorption or emission of light
Nuclear Magnetic Resonance (NMR) Spectroscopy
observes the chemical environments of the hydrogen atoms and provides evidence for the structure of the alkyl groups and clues to the functional groups.
Ultraviolet Spectroscopy
observes electronic transitions and provides information on the electronic bonding in the sample
frequency
The frequency of a wave is the number of complete wave cycles that pass a fixed point in a second. Frequency, represented by the Greek letter v (nu), is usually given in hertz (Hz), mean ing cycles per second
wavelength
represented by the Greek letter ,\ (lambda), is the distance between any two peaks (or any two troughs) of the wave.
relationship of wavelength and frequency
inversely proportional
v(lambda) = c
lambda= c/v
c= speed of light (3x10^10 cm/sec)
photons
massless packets of energy
Photon Frequency Calculation
E= hv
or E= hc/lambda
E= energy
h= planck's constant (6.62 x 10^-37 kJ/sec)
electromagnetic spectrum
range of all possible frequencies, from zero to infinity
electromagnetic spectrum list (longest wavelength/lowest frequency to shorter wavelength/higher frequency)
radio, microwave, IR, visible, near UV, vacuum UV, X rays, gamma rays
wavenumber (in cm^-1)
An analog of frequency used for infrared spectra instead of wavelength.
infrared spectrum
a graph of the energy absorbed by a molecule as a function of the frequency or wavelength of light
OH wavenumber
3200-3600
CH stretch
2850-3000
CO stretch
just above 1000
symmetric stretching
In IR spectroscopy, when two bonds are stretching in phase with each other.
A nonlinear molecule with n atoms generally has __________ fundamental vibrational modes.
A) 3n
B) 2n
C) 3n - 6
D) 3n - 3
E) 2n - 2
3n-6
n= atoms
fingerprint region
the region of the IR spectrum containing most of these complex vibrations ( 600 to 1400 em - I )
infrared spectrometer
measures the frequencies of infrared light absorbed by a compound. In a simple infrared spectrometer two beams of light are used. The sample beam passes through the sample cell, while the reference beam passes through a reference cell that contains only the solvent. A rotating mirror alternately allows light from each of the two beams to enter the monochromator.
carbon-carbon bond stretching frequencies
c-c 1200 cm- 1 c=c1660 cm- 1
C-=C <2200 cm-1
conjugated double bonds
double bonds separated by one single bond
effect of conjugated bonds
conjugated double bonds are slightly more stable than isolated double bonds because there is a small amount of pi bonding between them. This overlap between the pi bonds leaves a little less electron den sity in the double bonds themselves. As a result, they are a little less stiff and vibrate a lit tle more slowly than an isolated double bond
Carbon-hydrogen bond stretching
sp3 : 2800 to 3000
sp2 : 3000 to 3100
sp : 3300
alcohol and amine frequencies
OH 3300 broad
OH (acid) 3000 broad
NH 3300, with spikes
ketones, aldehydes, acids frequencies
ketone (R-c(=O)-R'): 1710
aldehydes (R-c(=O)-H): 1725
alcohol/carboxylic acid (O=C-O-H): 1710 =O and 2500-3500 for OH
primary amide
RCONH2
primary amine
R-NH2
secondary amide
RCONHR
secondary amine
R2NH
ketone
RCOR
aldehyde
CHO
ester
RCOOR
carbonyl
C=O
carboxylic acid
COOH
C-N frequencies
C-N : 1200
C=N : 1600
C=-N >2200
nitrile
RCN
imine
A double bond between a carbon and a nitrogen
mass spectroscopy
provides the molecular weight and valuable informa tion about the molecular formula, using a very small sample
mass spectrometer
ionizes molecules in a high vacuum, sorts the ions according to their masses, and records the abundance of ions of each mass. A mass spectrum is the graph plotted by the mass spectrometer, with the masses plotted as the x axis and the relative number of ions of each mass on they axis
ion source
the sample is bombarded by a beam of electrons. When an electron strikes a neutral molecule, it may ionize that mol ecule by knocking out an additional electron.
radical cation
A positively charged ion with an unpaired electron; commonly formed by electron impact ionization, when the impinging electron knocks out an additional electron
fragmentation
gives a characteristic mixture of ions
molecular ion (M+)
The positive ion formed in mass spectrometry when a molecule loses an electron.
m/z
mass to charge ratio
base peak
the most intense peak in a mass spectrum
molecular ion peak
The peak with the highest m/z ratio in the mass spectrum, the M peak
gas chromatograph
A device used to detect the composition of an unknown material
high-resolution mass spectrometer (HRMS)
t uses extra stages of electrostatic or magnetic focus ing to form a very precise beam and to detect particle masses to an accuracy of about 1 part in 20,000.
M+1 peak
A peak produced by a molecular ion with an increased mass due to the presence of one carbon-13 atom.
M+2 peak
an isotopic peak that is two mass units heavier than the major molecular ion peak
fragmentations often split off
simple alkyl groups
IR-active
a vibration that changes the dipole moment of the molecule and thus can absorb infrared light
IR-inactive
a vibration that does not change the dipole moment of the molecule and thus cannot absorb infrared light
nuclear magnetic resonance spectroscopy
most powerful tool available for organic structure determi nation. Like infrared spectroscopy, NMR can be used with a very small sample, and it does not harm the sample. The NMR spectrum provides a great deal of information about the structure of the compound, and many structures can be determined using only the NMR spectrum. More commonly, however, NMR spectroscopy is used in conjunc tion with other forms of spectroscopy and chemical analysis to determine the structures of complicated organic molecules.
magnetic moment
a measure of an object's tendency to align with a magnetic field
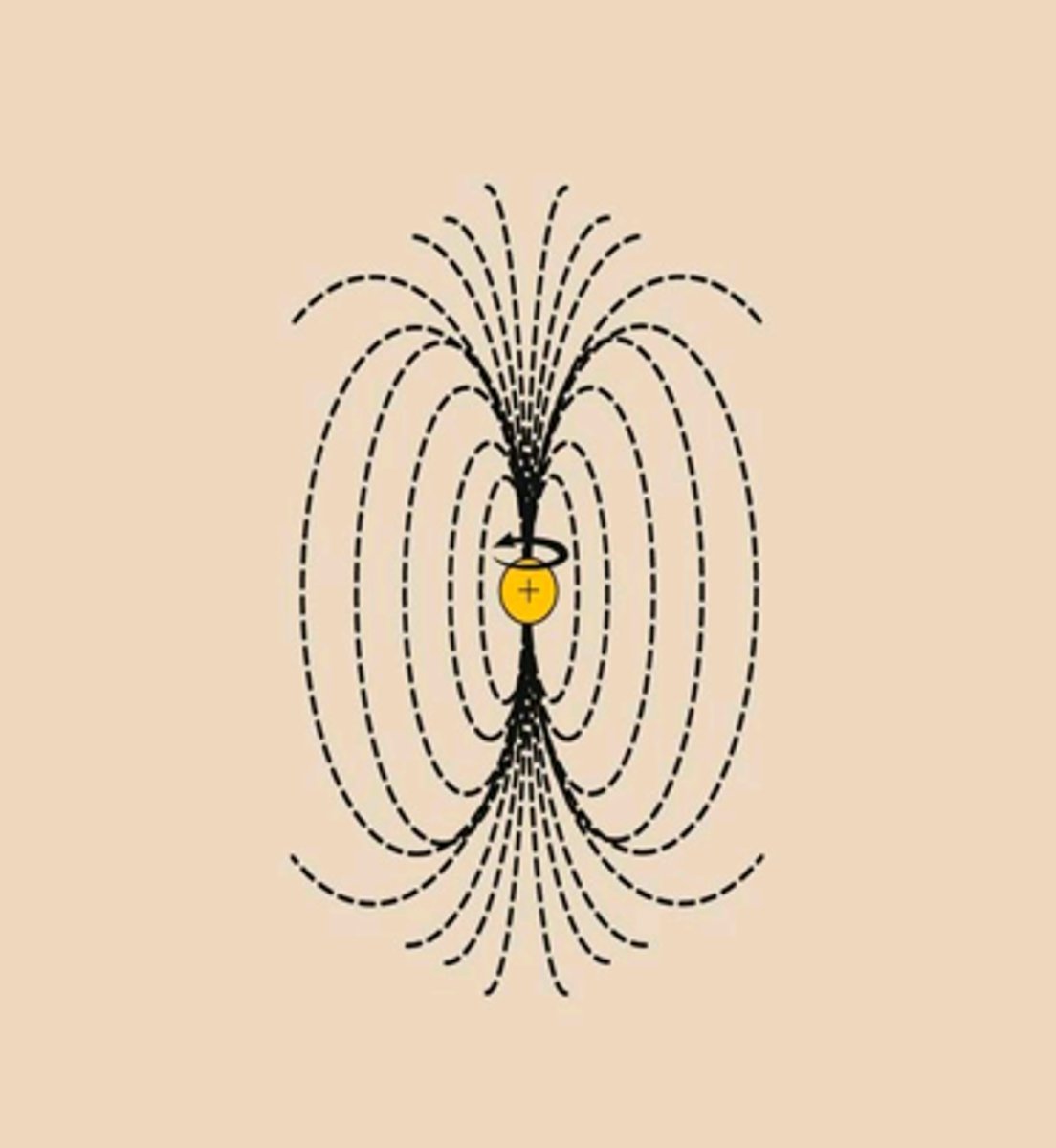
alpha spin state
Lower energy state with the proton aligned with the field
beta spin state
higher energy state with the proton aligned against the external magnetic field
without external magnetic field
proton magnetic moments have random orientations
delta E= y(h/2pi) B0
E = energy difference between a and beta states
h = Planck's constant
B0 = strength of the external magnetic field
y = gyromagnetic ratio, 26,753 sec- 1 gauss- ! for a proton
Gyromagnetic ratio
is a constant that depends on the magnetic moment of the nucleus under study. Magnetic fields are measured in gauss; for example, the strength of the earth's magnetic field is about 0.57 gauss. The SI unit for magnetic field is the tesla (T), which is simply 10,000 gauss.
e resonance frequency v is proportional to the
applied magnetic field ( B0) and the gyromagnetic ratio ( y)
induced magnetic field
the magnetic field set up by the motion of electrons in a molecule (or in a wire) in response to the application of an external magnetic field
shielded
A nucleus whose chemical shift has been decreased due to addition of electron density, magnetic induction, or other effects.
deshielded
A nucleus whose chemical shift has been increased due to removal of electron density, magnetic induction, or other effects.
Upfield (NMR)
to the right of the NMR spectrum
- protons experience more shielding
- higher magnetic field strength
Downfield (NMR)
LEFT. Deshielded by EWG or EN atom nearby.
chemical shift
The difference (in parts per million) between the resonance frequency of the proton being observed and that of tetramethylsilane (TMS).
tetramethylsilane (TMS)
In NMR data, all ppm values are referenced against what molecule?
chemical shift (ppm)=
shift downfield from TMS (Hz)/total spectrometer frequency (MHz)
TMS=
0 on scale of absorption
vinyl and aromatic protons
double bonds and aromatic rings produce large deshielding effects on their vinyl and aromatic protons. These deshielding effects result from the same type of circulation of electrons that normally shields nuclei from the magnetic field
Acetylenic hydrogens
Since the pi bond of an alkene deshields the vinyl protons, we might expect an acetylenic hydrogen (-C C-H) to be even more deshielded by the two pi bonds of the triple bond. The opposite is true: Acetylenic hydrogens absorb around 82.5, compared with 85 to 86 for vinyl protons. Figure 13-13 shows that the triple bond has a cylinder of electron density surrounding the sigma bond. As the mole cules tumble in solution, in some orientations this cylinder of electrons can circulate to produce an induced magnetic field
aldehyde protons
Aldehyde protons (-CHO) absorb at even lower fields than vinyl protons and aromatic protons: between 89 and 810. Figure 13-14 shows that the aldehyde proton is deshielded both by the circulation of the electrons in the double bond and by the inductive electron-withdrawing effect of the carbonyl oxygen atom.
hydrogen-bonded protons
The chemical shifts of 0-H protons in alcohols and N-H protons in amines depend on the concentration. In concentrated solutions, these protons are deshielded by hydrogen bonding, and they absorb at a relatively low field: about 83.5 for an amine N-Hand about 84.5 for an alcohol 0-H. When the alcohol or amine is diluted with a non-hydrogen-bonding solvent such as CC14, hydrogen bond ing becomes less important. In dilute solutions, these signals are observed around 8 2.
carboxylic acid protons
Because carboxylic acid protons are bonded to an oxy gen next to a carbonyl group, they have considerable positive character. They are strongly deshielded and absorb at chemical shifts greater than 810. Carboxylic acids frequently exist as hydrogen-bonded dimers (shown at right), with moderate rates of proton exchange that broaden the absorption of the acid proton.
The acid proton signal appears at a chemical shift that is not scanned in the usual range of the NMR spec trum. It is seen in a second trace with a 2.0 ppm offset, meaning that this trace corresponds to frequencies with chemical shifts 2.0 ppm larger than shown on the trace. The acid pro ton appears around 811.8: the sum of 89.8 read from the trace, plus the 82.0 offset.
chemically equivalent
Protons in identical chemical environments with the same shielding have the same chemical shift
tert-butyl acetoacetate has 3 types of protons, giving ____ signals in the NMR spectrum
three
The area under a peak is proportional to the number of
hydrogens contributing to that peak
ex: in the methyl tert-butyl ether spectrum (Figure 13-19), the absorp tion of the tert-butyl protons is larger and stronger than that of the methoxy protons because there are three times as many tert-butyl protons as methoxy protons.
CH3= 2 spaces
integrators (NMR)
compute the relative areas of peaks
___ / ____ = space per hydrogen
# spaces/ # hydrogens
theory of spin-spin splitting
splitting of signals into multiplets, called spin-spin splitting, results when two different types of protons are close enough that their magnetic fields influence each other. Such protons are said to be magnetically coupled.
magnetically coupled
nuclei that are close enough that their magnetic fields influence each other, resulting in spin-spin splitting
Spin-spin splitting is a reciprocal property. That is,
if one proton splits another, the second proton must split the first.
N + 1 rules
If a signal is split by N neighboring equivalent protons, it is split into N+ 1 peaks.
most spin-spin splitting is between
protons on adjacent carbon atoms
coupling constant
Distance between the peaks of a multiplet
complex splitting
splitting by two or more different kinds of protons with different coupling constants
diastereotopic
Substituents that have different chemical shifts in both achiral and chiral solvents
diastereomers
stereoisomers that are not mirror images
conformational changes
non-random movements triggered by the binding of a specific molecule
Carbon NMR
can only detect carbon-13, same as H NMR, but ignore splitting
interpreting CNMR
1. The number of different signals implies how many different types of carbons are present. 2. The chemical shifts of those signals suggest what types of functional groups con tain those carbon atoms. 3. The splitting of signals in the off-resonance-decoupled spectrum or the DEPT 90 and DEPT-135 spectra indicate how many protons are bonded to each carbon atom.
NMRI Nuclear Magnetic Resonance Imaging
Magnet linked to a computer creates images of inside the body
amine
R-NH2
primary secondary tertiary amines
Primary- N is connected to 1 C
Secondary- N is connected to 2 Cs
tertiary- N is connected to 3 Cs
quaternary ammonium salts
have four alkyl or aryl bonds to a nitrogen atom
how to name amines
list each alkyl group alphabetically that is bonded to the nitrogen followed by "amine"
aromatic and heterocyclic amines
generally known by historical names. Phenylamine is called aniline, for example, and its derivatives are named as derivatives of aniline.
nitrogen heterocycles
aziridine
pyrrole
pyrrolidine
1-methylpyrrolidine
imidazole
indole
pyridine
2-methylpyridine
piperidine
pyridine
purine