Section 1 - Atomic structure
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Protons
Location: nucleus
Relative mass: 1
Relaive charge: +1
Neutrons
Location: nucleus
Relative mass: 1
Relative charge: 0
Electrons
Location: outer shell
Relative mass: 1/1836
Relative charge: -1
Mass number (A)
Average mass of naturally occurring isotopes.
Total number of protons and neutrons.
Atomic number (Z)
Number of protons.
Isotopes
Same atomic number, different mass numbers.
Same electronic structure → same chemical properties.
Different mass numbers→ different physical properties.
Ions
Atoms form ions by gaining or losing electrons.
They are aiming for noble gas configuration.
+ = lost electrons
- = gained electrons
John Daltons model
Atoms are solid spheres.
Different spheres make up the different elements.
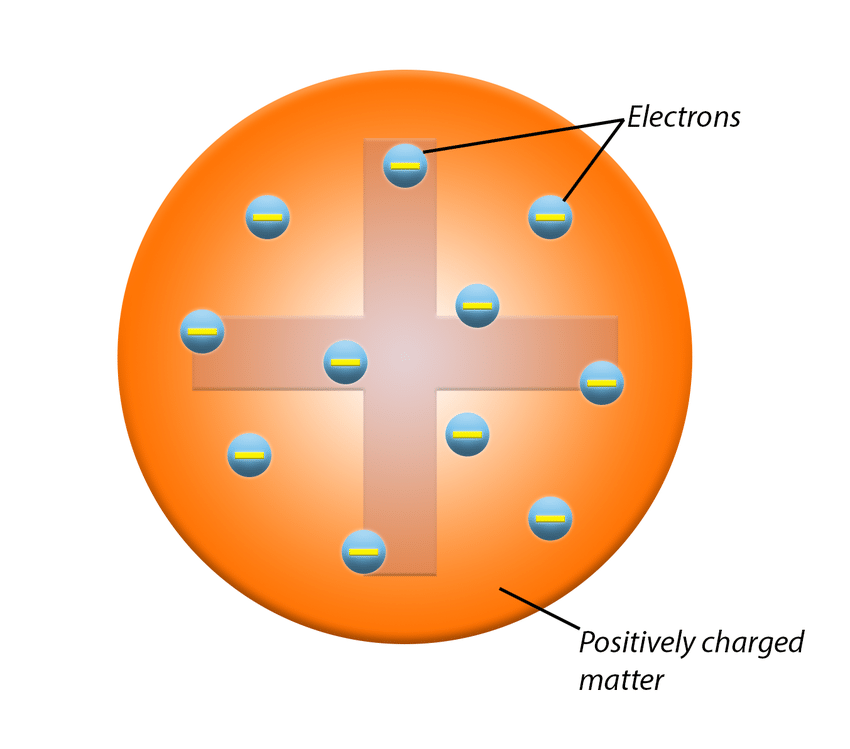
J. J. Thomsons model
Plum pudding model.
Atoms are electrons in a sphere of positive charge
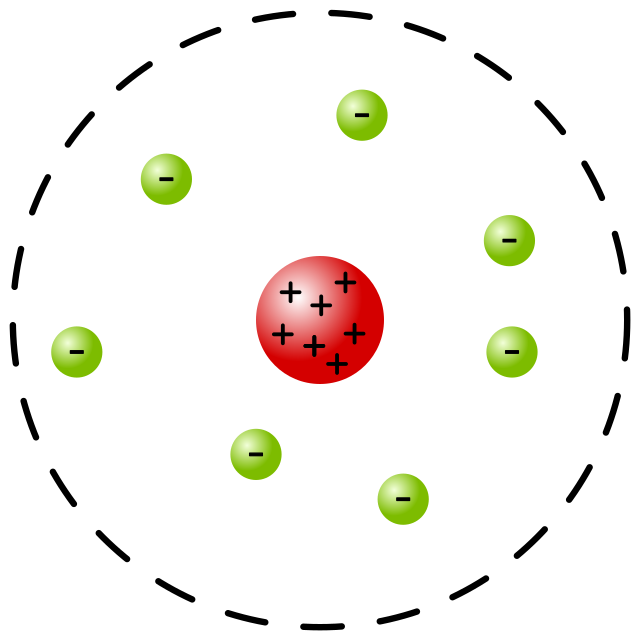
Ernest Rutherfords model
Gold foil experiment:
Fired positively charged alpha particles at a thin sheet of gold.
Most of the particles went through, very small number deflected backwards.
Most of the atom is empty space.
At the centre there’s a tiny positively charged nucleus, surrounded by a cloud of negative electrons.
Niehls Bohr model
Electrons only exist in fixed orbits around the nucleus and not anywhere in between.
Each orbit has a fixed energy.
When an electron moves between shells radiation is emitted or absorbed.
Absorbed when moving to a higher energy level (further from the nucleus)
Emitted when moving to a lower energy level (closer to the nucleus)
Because the energy levels are fixed, the radiation will have a fixed frequency.
Quantum model / modern model
Electrons exist in orbitals, not fixed paths.
These orbitals are grouped into sub shells within each shell.
Time of flight mass spectrometer can be used to?
To identify the different isotopes in a sample of an element.
4 things that happen when a sample is squirted into a time of flight mass spectrometer
1 - Ionisation (before sample enters the mass spectrometer)
Electron impact ionisation
Electrospray ionisation
2 - Acceleration
3 - Ion drift
4 - Detection
Electron impact ionisation
Sample is vaporised and high-energy electrons are fired at it from an electron gun.
This knocks off one electron from each particle, forming a 1+ ion (positive ion).
Electrospray ionisation
Sample is dissolved in a volatile solvent and pushed through via a fine needle.
A high voltage is applied to the needle, causing each particle to gain a H+ ion.
The solvent is then removed, leaving a gas made up of positive ions.
Acceleration
The positive ions are accelerated by an electric field.
The electric field gives the same kinetic energy to all the ions.
The lighter ions accelerate more because they’re lighter.
Ion drift
Next, the ions enter a region with no electric field.
They drift through it at the same speed as they left the electric field.
So the lighter ions will be drifting at higher speeds.
Detection
The detector detects the current created when the ions hit it and records how long they took to pass through the spectrometer.
This data is then used to calculate the mass/charge values needed to produce a mass spectrum.
Mass spectrometer notes
All the particles will have the same kinetic energy but the mass will determine the velocity.
Because lighter ions travel through the drift region at higher speeds, they reach the detector in less time than heavier ions.
Mass spectrometer - time taken for the ion to travel equation
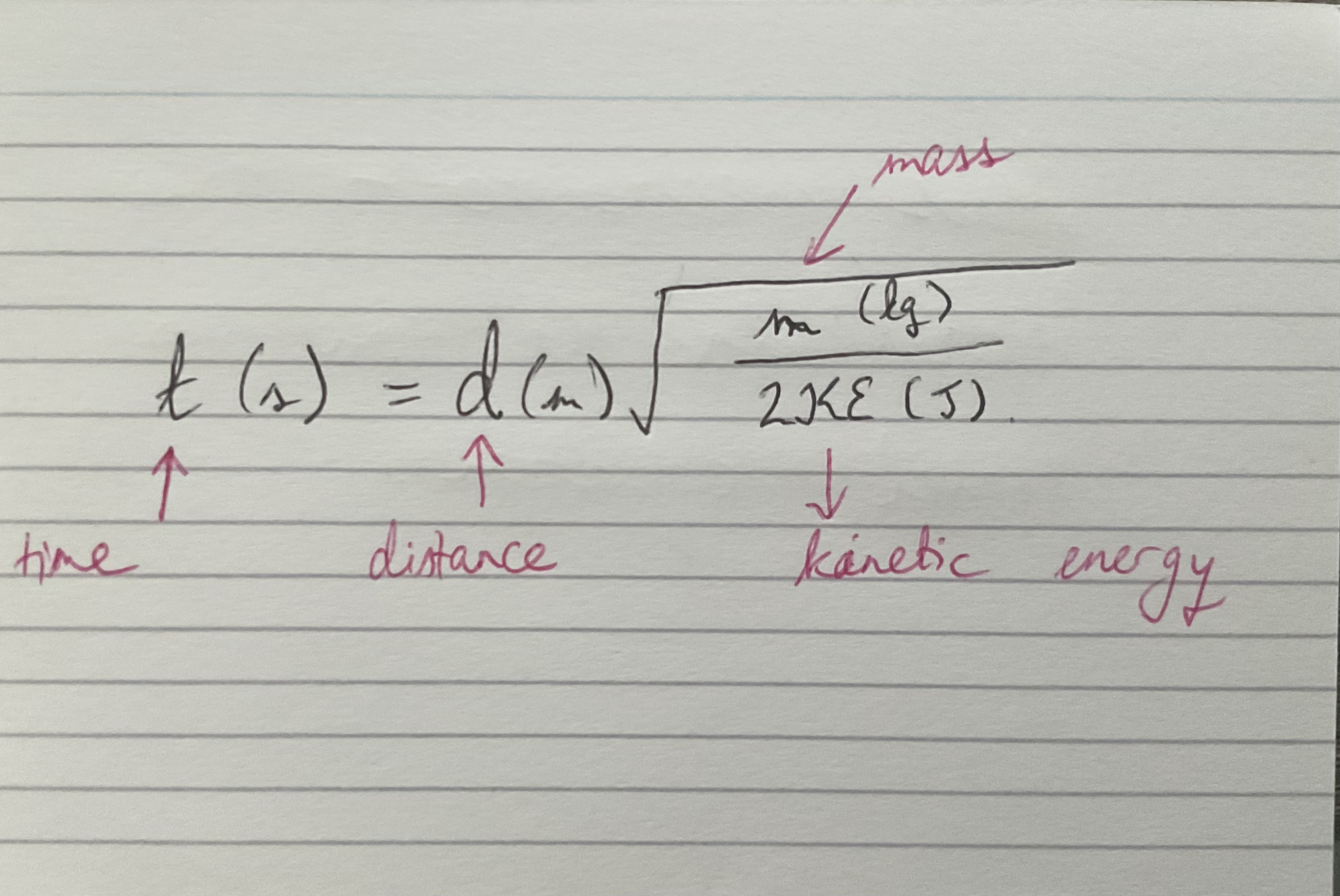
Electron shells
Electrons are arranged in shells.
1st shell contains one sub-shell, 1s.
2nd shell contains two sub-shells, 2s and 2p.
3rd shell contains three sub-shells, 3s, 3p and 3d.
The 4th shell contains four sub-shells, 4s, 4p, 4d and 4f.
Sub-shells
For atoms with more than one electron, shells are split into sub-shells that have slightly different energies.
The difference in energy between sub-shells is much less than the difference in energy between shells.
The sub-shells have different numbers of orbitals.
The 4s sub-shell has a lower energy level than the 3d sub-shell. This means the 4s sub-shell fills up first.
Orbitals
Sub-shells are composed of orbitals.
Orbitals in the same sub-shell have the same energy.
Each orbital can hold a maximum of two electrons.
An s sub-shell is made up of one s orbital.
A p sub-shell is made up of 3 orbitals.
A d sub-shell has 5 orbitals.
An f sub-shell has 7 orbitals.
Electron configuration rules
1 - Electrons fill up the lowest energy sub-shells first.
2 - Electrons fill orbitals in a sub-shell singly before they start sharing.
3 - For the configuration of ions from the s and p blocks of the periodic table, just add or remove the electrons to or from the highest energy occupied sub-shell.
Chromium and copper don’t follow the normal rules for electron configurations.
Chromium (Cr) and Copper (Cu) donate one of their 4s electrons to the 3d sub-shell.
Transition metals lose 4s electrons before their 3d electrons.
Electronic structure and chemical properties
The s block elements (Groups 1 and 2) have 1 or 2 outer shell electrons. These are easily lost to form positive ions with an inert gas configuration.
The elements in Group 5, 6 and 7 (p block) can gain 1, 2, or 3 electrons to from negative ions with an inert gas configuration.
Group 0 elements have completely filled s and p sub-shells and don’t need to gain, lose or share electrons. Their full sub-shells make them inert.
Groups 4 to 7 can also share electrons when they form covalent bonds.
Ionisation energy
Ionisation is the reaction where one electron is removed from an atom.
You must use the gas state symbol (g) because ionisation energies are measured for gaseous atoms.
Always refer to 1 mole of atoms rather than to a single atom.
The lower the ionisation energy, the easier it is to form a positive ion.
The first ionisation energy
The energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions.
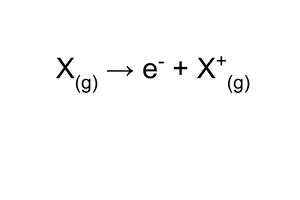
Second ionisation energy
The energy needed for the removal of one electron from each ion within one mole of 1+ ions in a gaseous form to make one mole of gaseous +2 ions.
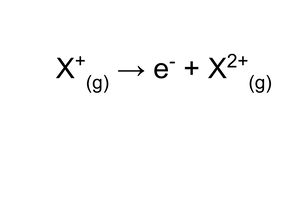
Second ionisation energy notes
Just like first ionisation energy, the value of second ionisation energy depends on nuclear charge, distance of the electron from the nucleus and the shielding effect of inner electrons.
Second ionisation energies are greater than first ionisation energies because the electron is being removed from a positive ion (and not an atom) which will require more energy.
The electron configuration of the atom will also play a role in how much larger the second ionisation energy is than the first.
Successive ionisation energies
You can remove all the electrons from an atom, leaving only the nucleus.
Each time you remove an electron, there’s a successive ionisation energy.
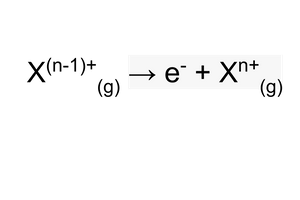
High ionisation energy
A high ionisation energy means there’s a high attraction between the electron and the nucleus, so more energy is needed to remove the electron.
3 things that affect ionisation energy
1 - Nuclear charge
2 - Distance from nucleus
3 - Shielding
Nuclear charge
The more protons there are in the nucleus, the more positively charged the nucleus is and the stronger the attraction for the electrons.
Distance from nucleus
Attraction falls of very rapidly with distance.
An electron close to the nucleus will be much more strongly attracted than one further away.
Shielding
As the number of electrons between the outer electrons and the nucleus increases, the outer electrons feel less attraction to the nucleus.
This lessening of the pull of the nucleus thanks to the inner electron shells is called shielding.
Ionisation energies and shell structure
A graph of successive ionisation energies provides evidence for the shell structure of atoms.
If you know the successive ionisation energies of an element you can work out the number of electrons in each shell of the atom and which group the element is in.
Within each shell, successive ionisation energies increase. This is because when you remove the first electron, the atom becomes a positive ion.
When you try to remove the next electron after the first ionisation energy:
The atom is more positively charged, so it pulls on the remaining electrons more strongly.
There are fewer electrons, so there’s less repulsion between them (even if electrons are just in the same shell, they still repel each other).
This means that the remaining electrons are held more tightly by the nucleus.
So it takes more energy to remove each one after the last.
Trends in first ionisation energies - across a period
First ionisation energy increases across a period.
This is because the atomic radius decreases. Proton number increases across a period, so electrons are more attracted to the nucleus.
The electron is closer to the nucleus, and so experiences a greater attraction.
Nuclear charge increases across a period.
Trends in first ionisation energies provides - down a group
First ionisation energy decreases down a group.
This is because the atomic radius increases. The electron is further from the nucleus, and so experiences lower attraction.
There are also more electrons between the nucleus and the outer electrons. This means outer electrons experience greater shielding from the nucleus.
This also provides evidence for the existence of shells.
Relative molecular mass (Mr) definition
The average mass of a molecule compared to 1/12 the mass of one atom of carbon.
Relative atomic mass (Ar) definition
The average mass of one atom compared to 1/12 the mass of one atom of carbon.
Relative atomic mass equation
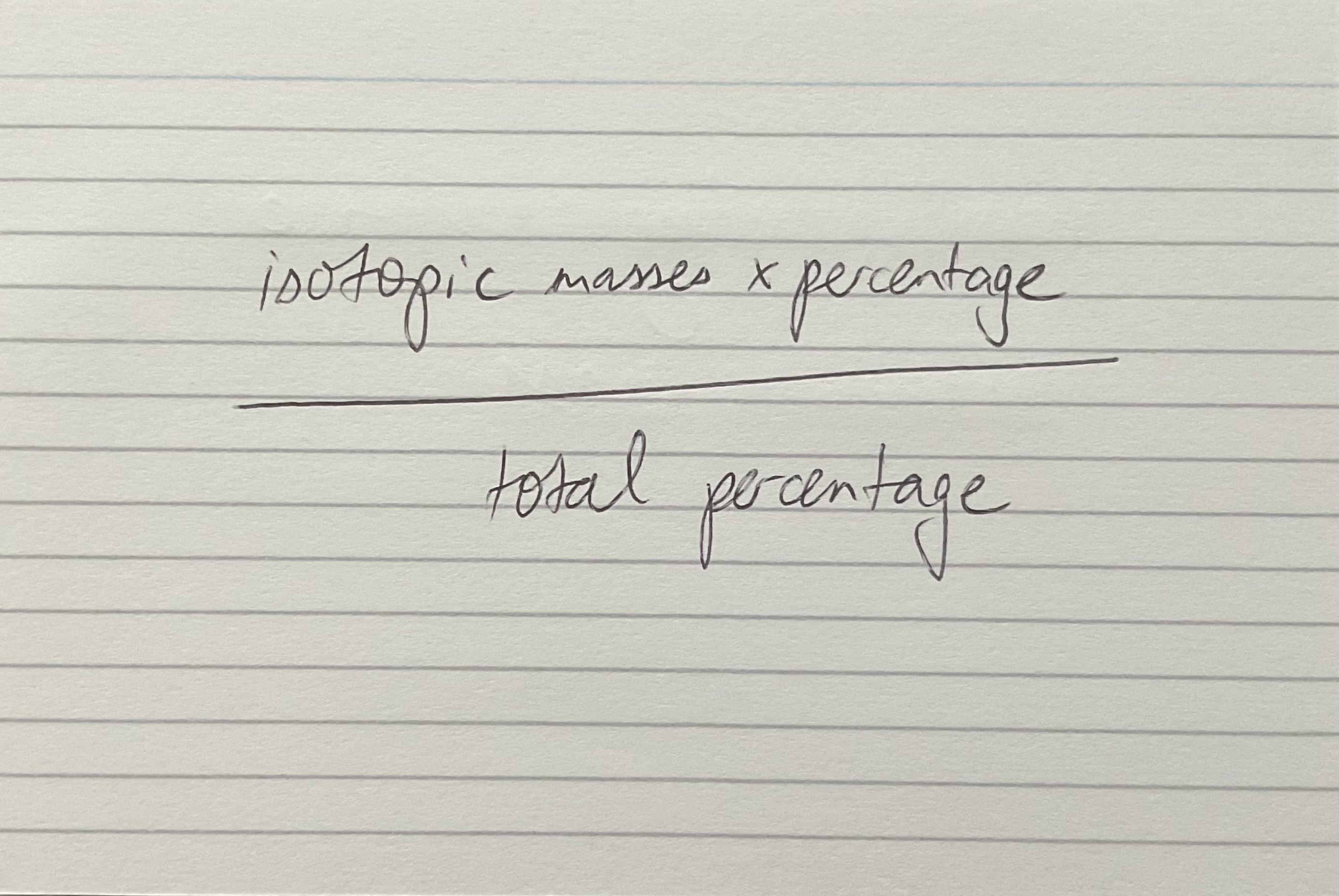
Relative molecular/formula mass equation
Add up the relative atomic masses (At) of all atoms in the substance.
Explain why electrons do not spiral into the nucleus, despite being negatively charged and attracted to the positively charged nucleus.
Because they occupy shells.
An electron can only exist in specific energy states and cannot exist between them.
This is explained by Bohrs model and later by quantum mechanics.
Why do we assume atoms have the same number of protons and electrons?
Because atoms are electrically neutral, so the total positive charge from protons must equal the total negative charge from electrons.
Why do chromium (Cr) and copper (Cu) have anomalous electron configuration?
Half-filled (Cr) or fully filled (Cu) d-subshells are more stable.
Electron shifts from 4s → 3d to achieve extra stability.
How does the number of outer electrons determine chemical bonding and reactivity?
Outer electrons determine how many bonds an atom can form.
Atoms gain, lose or share lecterns to achieve a full outer shell.
Few outer electrons → likely to lose electrons → form cations.
Many outer electrons → likely to gain electrons → form anions.
Bond type depends on electron sharing (covalent) or transfer (ionic).
Reactivity is influenced by how easily atoms can achieve a full outer shell.
How was the mass of protons and neutrons determined?
By mass spectrometry and nuclear reactions.
How was the mass of electrons determined?
Using Millikans oil drop experiment + Thomsons cathode ray experiment.