Chemistry Section 7: The Quantum-Mechanical View of the Atom and Periodicity
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Electromagnetic Radiation (EMR)
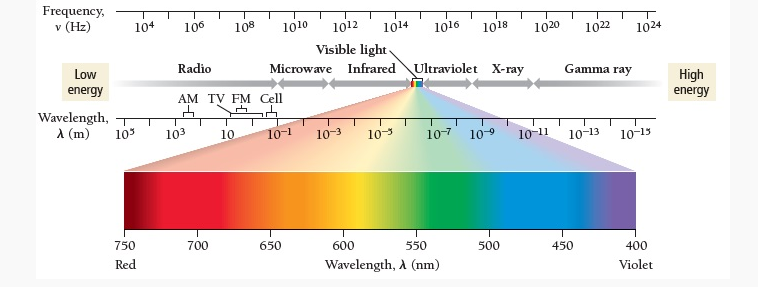
Energy (in the form a “light”) travels through space by electromagnetic radiation
there are 7 types of EMR which togteher make up the electromagnetic spectrum
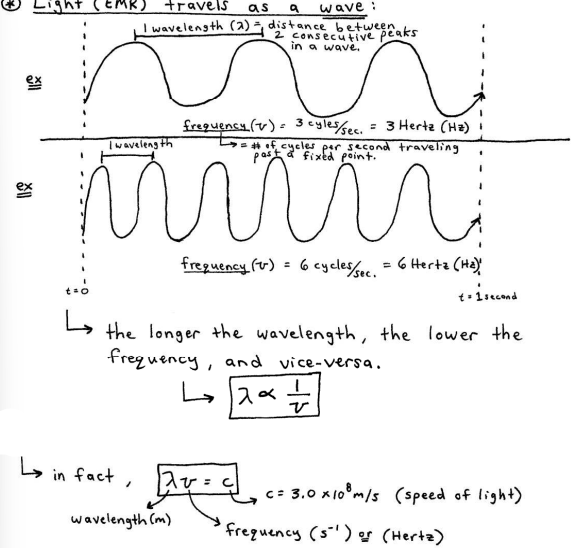
light (EMR) only differs in its energy, frequency, and wavelength
visible light only makes up a “sliver) of the spectrum
remember the complimnetary colors for visible light, use (and sound out) “VY BO GR”
Light (EMR) travels as a wave
The Nature of Matter
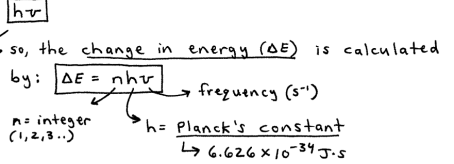
in the early 1900’s, Max Planck discovered that matter cannot absorb or emit any amount of energy. Instead,he postulated that energy can only be gained or lost in whole-number mulitpies of the quanitiy
because energy is transferred (absorbed or emitted) in these finite quantites, or “packets of energy”, the energy is said to be quantized (only occurs in discrete units of size)
The wave-particle duality of light
phenomenon in which light can exhibit both wave-like and particle-like behavior
The energy of a photon is given by

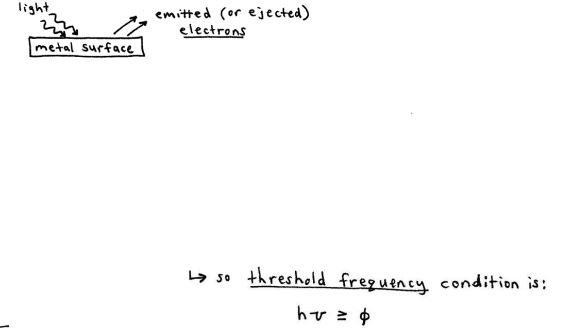
Photoelectric Effect
the observation that many metals emit electrons when light shines upon them
the light used to dislodge the electrons must have a high enough energy such that it meets or exceeds the threshold freguency
a single photon must have enough energy to dislodge an e- which is bound to the metal with a binding energy
The Atomic Spectrum of Hydrogen, and the Bohr Model of Hydrogen.
know that a “continous spectrum” is a rainbow-like spectrum that occurs when a prism diffracts ("splits") white light.
when a H atom absorbs energy (hv), its single becomes excited and moves to a higher energy state. when the excited electron later "falls back"(or relaxes) to its ground state, the electron releases the absorbed energy by emitting light of various wavelengths.
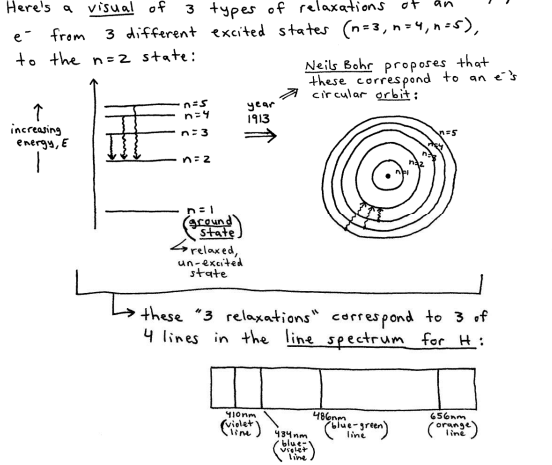
Bohr’s circular orbit model
the e cannot be found "just anywhere", but instead enly in those n=1,2,3... energy-level orbits.
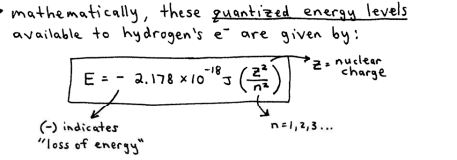
Quantum Numbers
the location of an electron in an atom is given by its set of four quantum numbers
n, l, ml,ms
an electron's complete set of 4 quantum #'s are its address (where it "lives" or "resides" in the atom)
no two electrons in an atom can have the same set of all 4 quantum #'s, Each e has a unique set (address).
Principal Quantum # (n)
n = 1,2,3…
reflects the size and energy of the atomic orbital that the e- is contained in
as n increases the orbital becomes larger and the e spends more time further from the nucleus.
as n increases the e- becomes higher in energy because, being farther from the nucleus, its energy is “less negative”
Atomic orbital
a region of space that gives an electron’s location as a “probable distribution’ of where it (the e-) can be found
Angular Momentum Quantum # (l)
l = 0,1,2… (n-1), for each value of n
tells you the shape of the orbital that the e- resides in
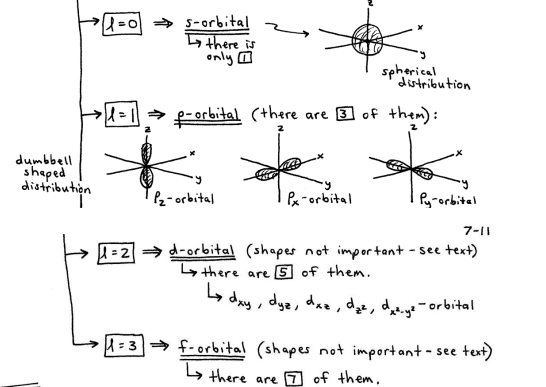
Magnetic Quantum # (ml)
ml = {-l,….o, ….+l}
the total # of ml values tells you the total # of orientations that particular atomic orbital has in 3-D space
Electron Spin Quantum # (ms)
ms = +1/2 or -1/2 (spin up and spin down)
became necessary because the "Pauli Exclusion Principle" which states that in a given atom, no 2 electrons can have the same set of 4 quantum numbers.
Polyelectronic Atoms (atoms other than hydrogen)
the Aufbay Principle states that electrons prefer to be in the lowest-energy level possible, so they will "fill in" to the lowest-E atomic orbital that's available
a single atomic orbital can hold a maximum of 2e-. sany type (1s, 3p, 6f, 4d, etc...)
thus, e's fill the orbitals of an atom and setle in to their own unique address" like this:
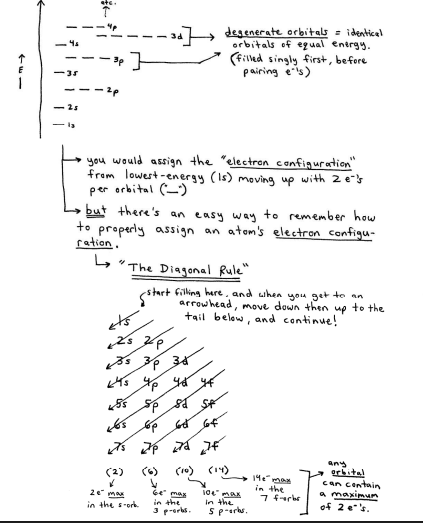
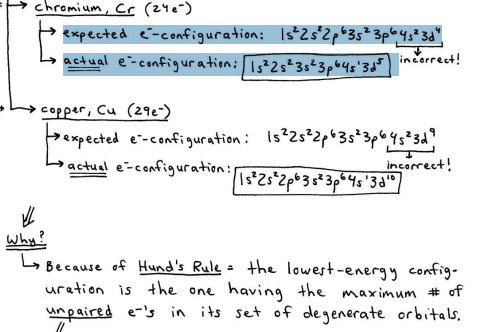
2 exceptions to the Diagonal Rule: Cr, Cu
Core electron vs. Valence Electrons
core electrons = the e-'s "buried" within the interior of the atom. Too "hidden" to be involved in bonding.
valence electrons = the e-'s in the outermost principal quantum energy-level (n). These are the electrons involved in bonding.
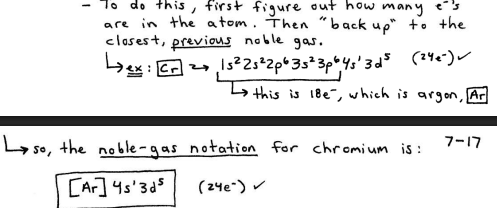
Noble-gas notation
Atomic Radius (Atomic Size)
half the distance between 2 nucleii in a molecule consisting of identical atoms.
atomic radii are measured this way because the size of an atom cannot be specified exactly or directly.
the "outer e cloud" is not well-defined.
across a period left to right = atomic size gets smaller because the increasing nuclear charge (more protons in nucleus) outweighs the additional e
down a group = atomic size gets larger because the increasing nuclear charge doesn’t outweigh the increasing # of electrons and the increased energy levels (n)

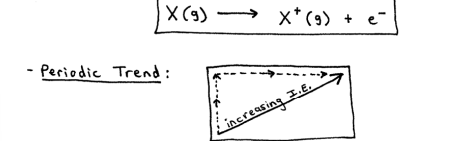
Ionization Energy
the energy required to remove an electron from a gaseous atom or ion
the larger the size of the atom or ion, the easier it is to remove an e- because it’s further away from the positively charged nucleus (contains protons). The easier it is to remove an e-, the less energy that’s required
increase for 2 reasons:
removing an e- that is often (not always) closer to the positively-charged nucleus.
removing an e- from an ion with more overall positive charge
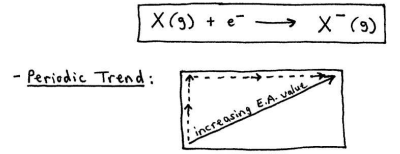
Electron Affinity
the energy that gets released when an atom gains an e-
the more negative the amount of energy, the greater the amount of energy being released