BioChem Lab final part 1
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
What is the stationary phase in SEC?
Mass of beads within the column
A spectrophotometer measures
A solutions absorbance at a specific wavelength
What did we use in the Protein Fractionation lab to separate proteins?
An acid and a salt
Why do set pipettes to maximum volume?
To preserve the internal spring
In the traditional method of pipetting, you collect the liquid while pressing at the:
First stop
0.777 ml is ____ uL
777.0
You can use P ___ to collect 0.2ml of solution
P2
___is the unit used to identify protein size
Daltons
In SEC, the biomolecules that are larger than the exclusion limit elutes out
Larger elutes first
In traditional method of pipetting, you collect the liquid while pressing to the ___ stop
First
___ is the type of chromatography where the column is filled with beads coated in "ligand"
Affinity
___ is the type of chromatography that is separation of proteins based on charge attraction and repulsion
Ionic exchange
Give one example of systematic error:
Pipettes are incorrectly calibrated
Give one example of a non-systematic error:
user errors such as wrong measurements
Typically ___ error is constant and can always be accounted for
Systematic
The ___ phase is usually the liquid phase that travels through the stationary phase pulling the compounds and separating them in column chromatography
Mobile
A protein has the lowest solubility at their ___ point
isoelectric
Salting our depends on the ___ of a protein
charge
Only ___ -toe shoes are acceptable to wear in the lab
closed
The ___ molecules move through the column first
Largest
Your labs are intended to expose you to concepts and methods of research and not to produce perfect results
True
Biuret assay is comprised of one redox reduction
True
When putting the pipette away, it should be set to its minimum setting
False
As soon as you collect the sample you are not supposed to allow the plunger to snap back in to position
True
After you plot your standard curve, you can only find the concentrations of unknown samples within the range of the curve
True
In affinity chromatography, it is important to use a buffer made of high concentration of salts or acid to finally elute out the protein of interest.
True
Running a blank is not necessary when you have to find the concentration of just one protein using a spectrophotometer
False
Forward pipetting is the best pipetting method to use when dealing with a viscous solution
True?
We keep proteins cold to prevent denaturing them
True
Non-systemic error is hard to account for as it is variable and usually caused by carelessness on the experimenter's part
True
SEC the liquid solution containing the protein sample, larger molecules elucidate first
mobile phase
SEC beads with pores trapping small proteins
stationary phase
The liquid is drawn while the plunger is at the second stop. This is to fully pull up the liquid and prevent the uptake of air
Reverse pipetting
A specific ligand is used as the stationary phase to bind to the desired protein
Affinity chromatography
Uses beads of the opposite charge of the desired protein
Ion exchange chromatography
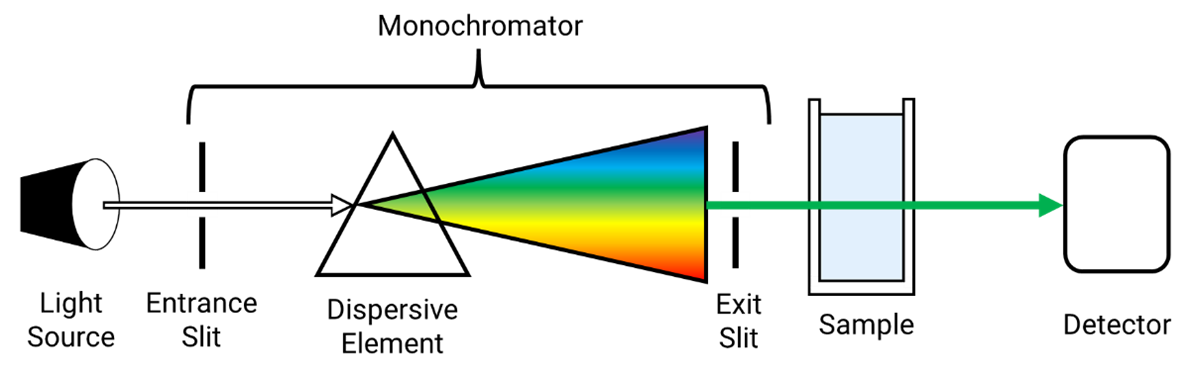
Diagram of UV-spectrometer
Light source, entrance, dispersive element, wavelength, exit, sample, detector
If your pipette is giving inconsistent results, what could be the reason
Pushed to the second stop, side ways, incorrectly calibrated