5.Preparation of Haloarenes
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Aryl chlorides and bromides
These compounds can be easily prepared from hydrocarbons by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride.
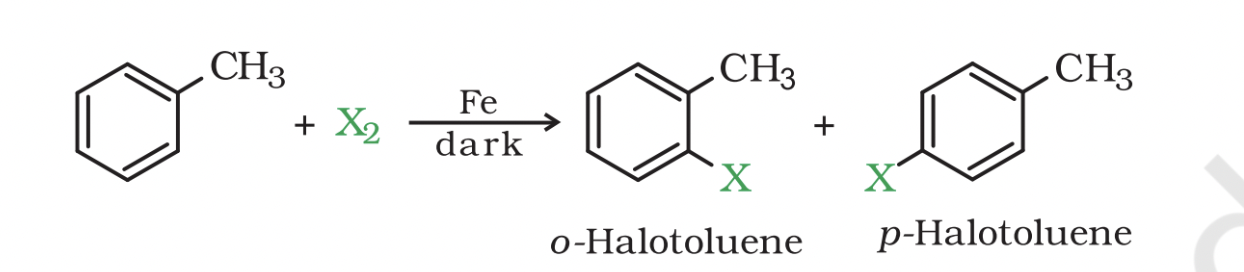
Ortho and para isomers
The ortho and para isomers of aryl chlorides and bromides can be easily separated due to a large difference in their melting points.
Reactions with iodine
Reactions with iodine are reversible in nature and require the presence of an oxidizing agent (HNO3, HIO4) to oxidize the HI formed during iodination.
Fluoro compounds
Fluoro compounds are not prepared by the method of electrophilic substitution due to the high reactivity of fluorine.
Sandmeyer's reaction
Amines can be converted into diazonium salts through Sandmeyer's reaction, where a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite.
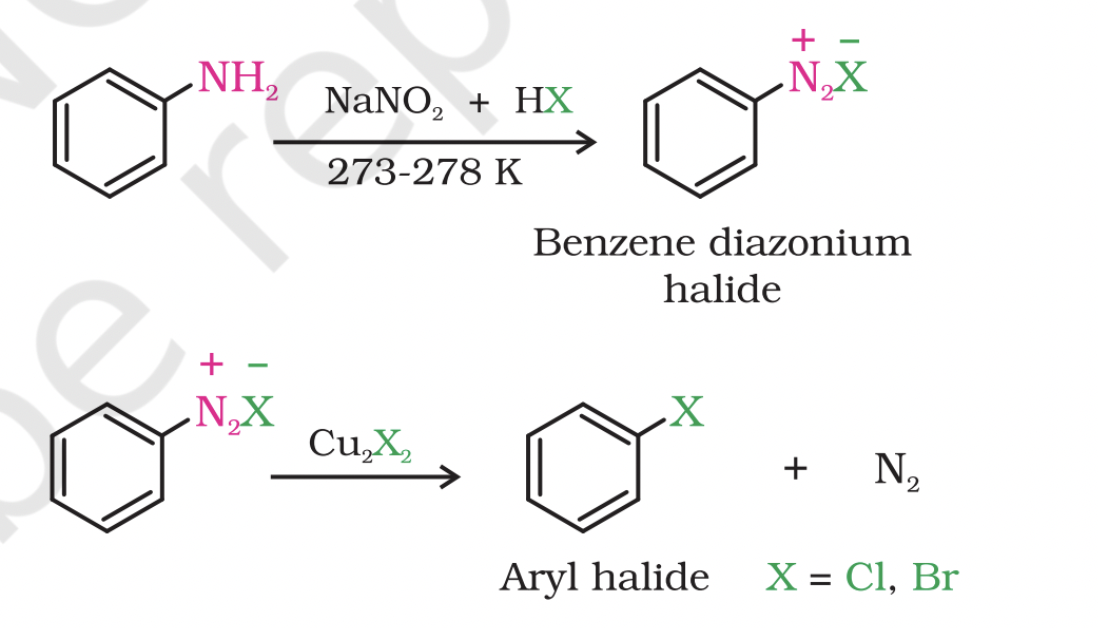
Diazonium salt
Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group by -Cl or -Br.
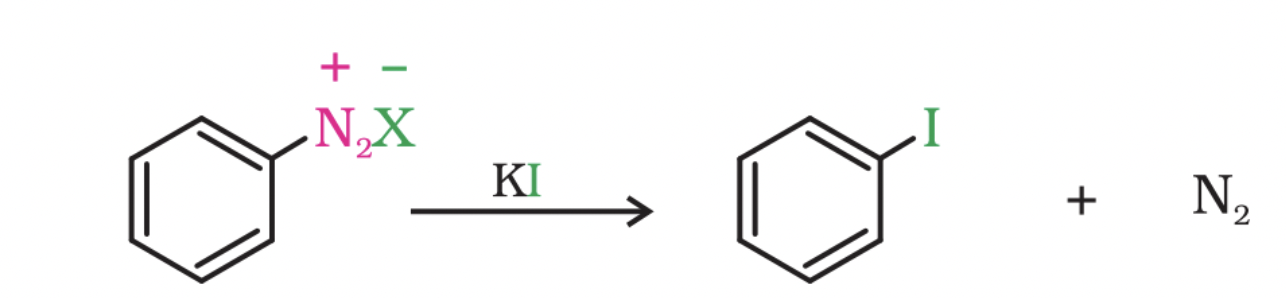
Replacement of diazonium group by iodine
The replacement of the diazonium group by iodine does not require the presence of cuprous halide and is done simply by shaking the diazonium salt with potassium iodide.