Chapter 9: Cellular Respiration and Fermentation
1/55
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
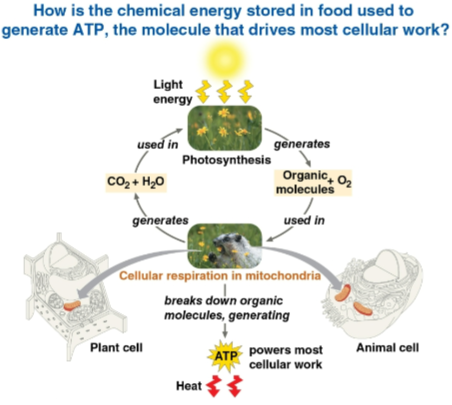
Cellular respiration
The process by which plant and animal cells break down organic molecules within mitochondria
Uses O2 and organic molecules to make ATP with waste CO2 and H2O
Includes aerobic and anaerobic respiration
Photosynthesis
The use of light, CO2, and H2O to make organic molecules and O2
Catabolic pathways
Chains of exergonic reactions that release stored energy by breaking down complex molecules using electron transfers from food molecules
Only linked to work by ATP as they do not power work
Fermentation
A partial degradation of sugars that occurs without oxygen; is a type of anaerobic respiration
Aerobic respiration
Process that utilizes oxygen and organic molecules to yield ATP
Represented by the equation: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Energy takes the form of ATP and heat
Electron transfer
The transfer of electrons during chemical reactions to release stored energy in organic molecules for ATP synthesis
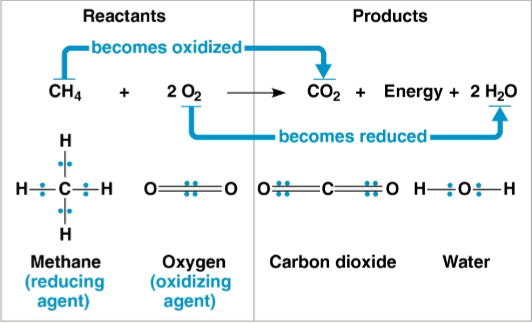
Redox reactions (reduction-oxidation reactions)
Chemical reactions that transfer electrons between reactants
Some electrons are shared via covalent bonds in these
Oxygen atoms attract electrons and do not share them equally; this still constitutes one of these
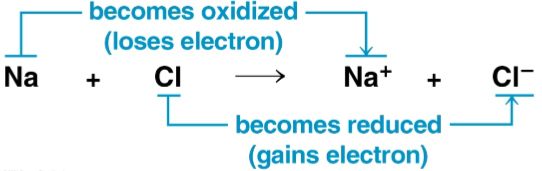
Oxidation
The loss of electrons from a substance in a redox reaction
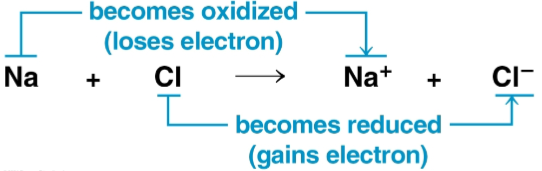
Reduction
The addition of electrons to a substance in a redox reaction
Refers to the positive charge
Reducing agent
The electron donor in a redox reaction
Reduces the electron acceptor
Seen with food molecules in cellular respiration due to their high abundance of hydrogen
Oxidizing agent
The electron acceptor in a redox reaction
Oxidizes the electron donor
Seen with oxygen during cellular respiration
Electronegativity
The attraction of electrons toward an atom; higher levels of this attract more electrons
Electrons lose potential energy when shifting to atoms with higher levels of this, releasing kinetic energy for ATP synthesis
Nicotinamide adenine dinucleotide (NAD+)
A coenzyme that functions as an electron carrier and oxidizing agent during cellular respiration
NADH
The reduced form of NAD+, representing stored energy tapped to synthesize ATP
Passes electrons to the electron transport chain through a series of redox reactions, releasing a small amount of energy
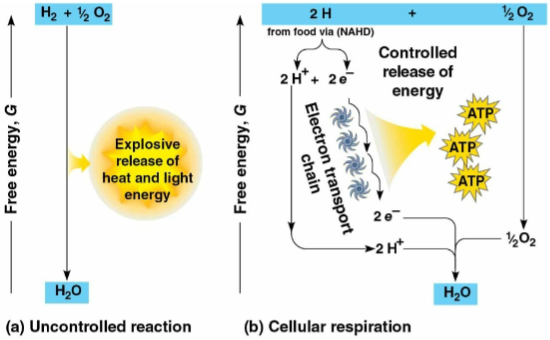
Electron transport chain
A series of molecules built into the inner membrane of the mitochondria to break the fall of electrons to oxygen in several energy-releasing steps
Oxygen
The final electron acceptor in the electron transport chain that captures electrons and hydrogen nuclei to form H2O
Yields energy through the attraction of electrons in redox reactions
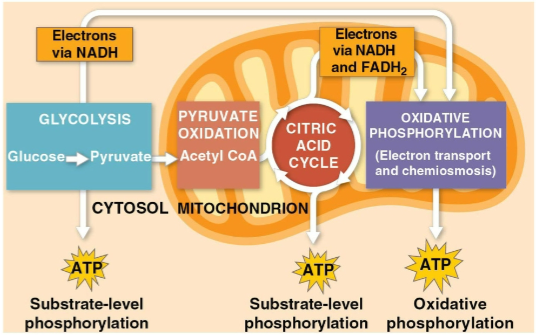
Glycolysis
The first step of cellular respiration, breaking down one molecule of glucose into two molecules of pyruvate
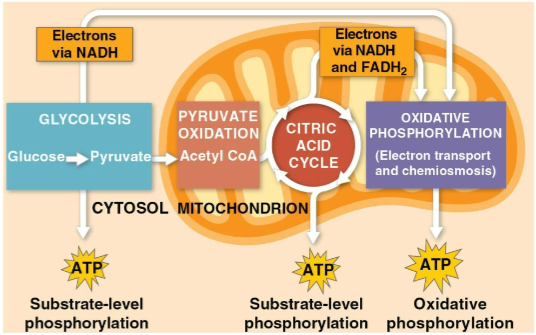
Citric acid cycle
The second step of cellular respiration with pyruvate oxidation, completing the breakdown of glucose to CO2
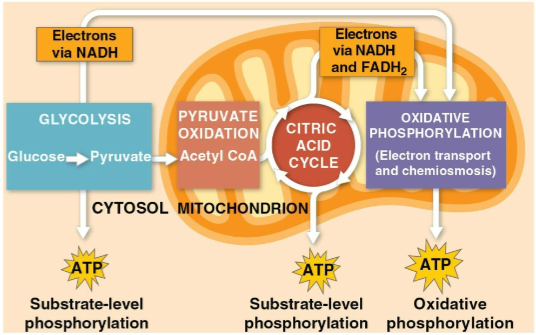
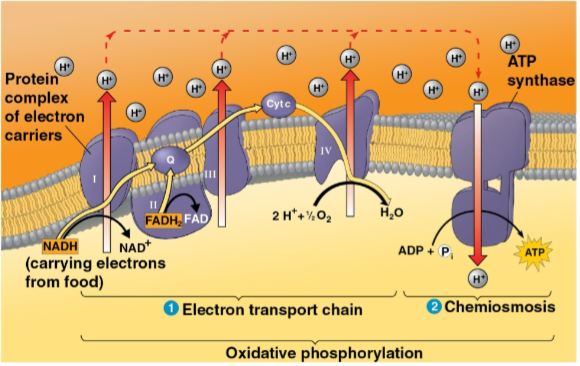
Oxidative phosphorylation
The closing of cellular respiration and the electron transport chain to facilitate synthesis of 90% of the cell’s ATP
Powered by redox reactions
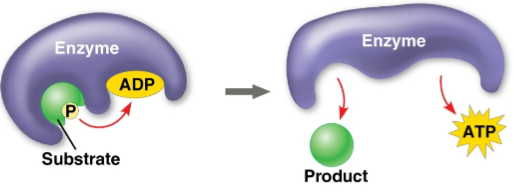
Substrate-level phosphorylation
The formation of some ATP in glycolysis and the citric acid cycle after an enzyme transfers a phosphate group directly from a substrate to ADP
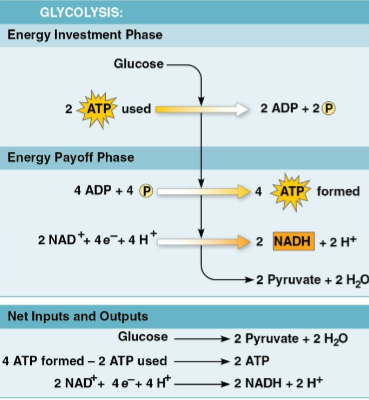
Glycolysis
The breakdown of one glucose molecule to two molecules of pyruvate that occurs in the cytoplasm
Creates two net ATP molecules by substrate-level phosphorylation
Done through an energy investment and energy payoff phase
Does not release any CO2, occuring whether or not O2 is present
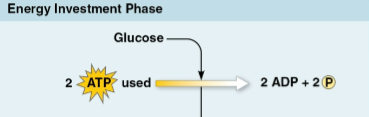
Energy investment phase
The first phase of glycolysis, splitting glucose into two three-carbon sugar molecules (G3P) using 2 molecules of ATP
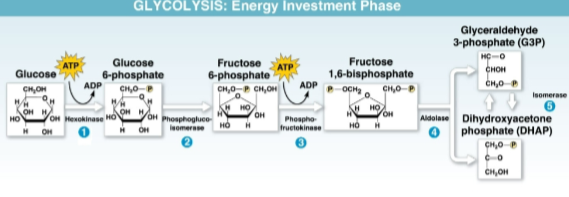
Glyceraldehyde 3-phosphate (G3P)
The three-carbon sugar molecule that glucose is initially broken into two of in the energy investment phase using 2 molecules of ATP
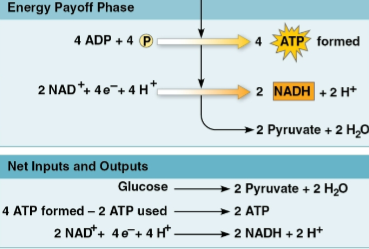
Energy payoff phase
The second phase of glycolysis
Synthesizes 4 molecules of ATP
Reduces 2 NAD+ to NADH
Oxidizes the small sugars to create 2 pyruvate and 2 H2O
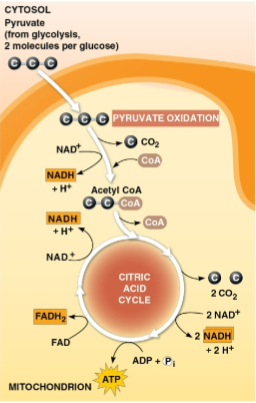
Pyruvate
Molecules created as a product of glycolysis in the first step of cellular respiration to be passed into processes for pyruvate oxidation and the citric acid cycle
Pyruvate oxidation
The removal of electrons from a pyruvate molecule before entering the citric acid cycle with most of the energy from glucose remaining
Happens within a mitochondrion if oxygen is present, or within the cytosol for aerobic prokaryotes
Coenzyme A
The coenzyme that pyruvate is joined to to form acetyl CoA after pyruvate oxidation
Acetyl coenzyme A (Acetyl CoA)
The substance pyruvate is converted to before entering the citric acid cycle
Pyruvate dehydrogenase
An enzyme that breaks down pyruvate through the catalyzation of three reactions
Oxidation of pyruvate’s carboxyl group, releasing the first CO2 of cellular respiration
Reducation of NAD+ to NADH
Combination of the remaining two-carbon fragment with coenzyme A to form acetyl CoA
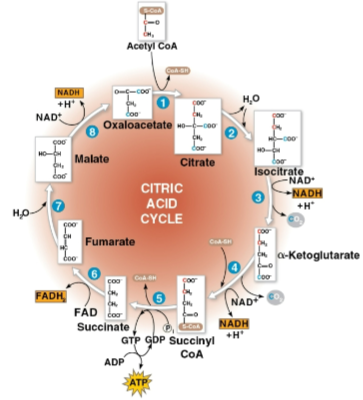
Citric acid cycle (Krebs cycle)
Cycle that oxidizes organic fuel derived from pyruvate, generating 1 ATP, 3 NADH, and 1 FADH2 per turn as well as 2 CO2 as waste
Runs twice per glucose molecule consumed due to 2 pyruvate being present after glycolysis
Has eight steps, each catalyzed by a specific enzyme, to join acetyl CoA and oxaloacetate to form citrate and then decompose it back to oxaloacetate for a cycle
NADH and FADH2 carry electrons to the electron transport chain
Oxaloacetate
The molecule that acetyl CoA is joined with to form citrate and is eventually decomposed back to after the citric acid cycle
Citrate
The molecule that results when joining acetyl CoA and oxaloacetate before decomposing back into oxaloacetate
NADH and FADH2
The two electron carriers produced by the citric acid cycle to carry electrons to the electron transport chain
NADH and FADH2
The two electron carriers produced during glycolysis and the citric acid cycle that account for most of the extracted energy from glucose
These donate electrons to the electron transport chain, powering ATP synthesis
Inner mitochondrial membrane
The location of the molecules of the electron transport chain in eukaryotic cells, folded into cristae for greater surface area
Mostly comprised of proteins as part of a multi-protein complex that accepts electrons
Plasma membrane
The location of the electron transport chain in prokaryotic cells
Cytochromes
Proteins with heme groups containing an iron atom
Serves as one of the carrier molecules in the electron transport chain
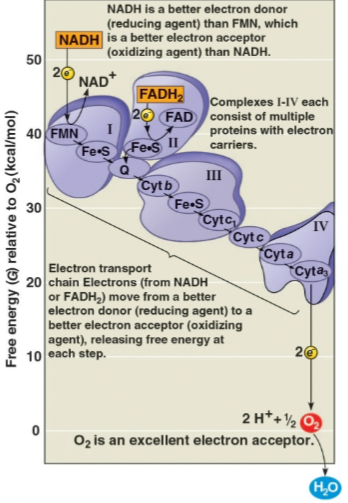
Electron transport chain
The chain of molecules that passes on electrons from NADH and FADH2 through proteins to gradually release free energy towards oxygen molecules
Serves to make energy to pump H+ from the mitochondrial matrix to the intermembrane space to catalyze ATP synthesis
Can also accept and release H+ to maintain the H+ gradient and couple reactions to ATP synthesis
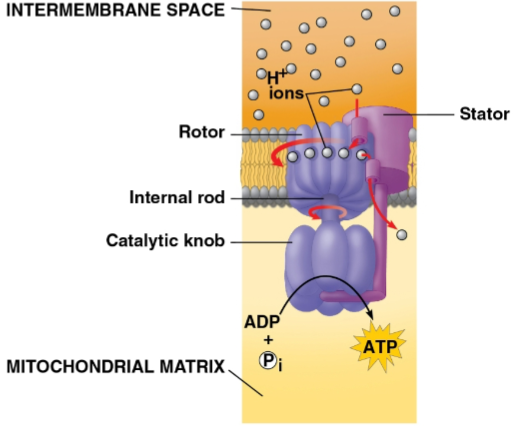
ATP synthase
The protein pump that H+ moves across after being powered by electrons to move into the intermembrane space
The movement of H+ down the concentration gradient onto this protein’s binding sites in a rotor causes a spin that catalyzes ADP phosphorylation
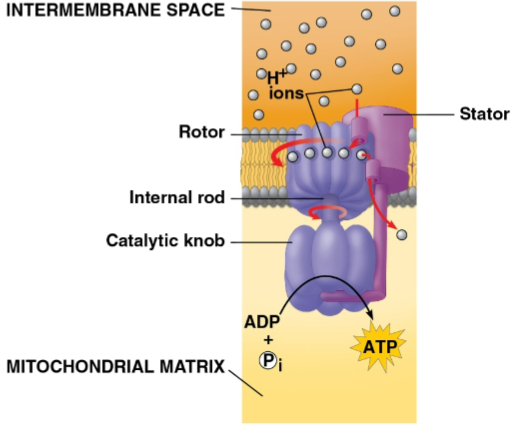
Chemiosmosis
The use of energy in a H+ gradient to drive cellular work
Seen in oxidative phosphorylation where H+ atoms are moved into the intermembrane space by electrons then moved into an ATP synthase rotor, catalyzing phosphorylation
Proton-motive force
An H+ gradient with the capacity to do work
32
The approximate number of ATP molecules created as a result of cellular respiration, representing about 34% of the energy in a glucose molecule
The rest is lost as heat
Aerobic respiration
The most common type of cellular respiration that involves the use of oxygen to pull electrons down the electron transport chain
Anaerobic respiration
Respiration wihtout oxygen that uses an electron transport chain with an electron acceptor other than oxygen
Sulfate ions may serve the role of acceptor in some organisms, making H2S instead
Glycolysis
Process that oxidizes glucose to pyruvate without the involvement of O2 or an electron transport chain
Produces 2 net ATP by substate-level phosphorylation regardless of O2 presence
Most widespread catabolic pathway on Earth that functions in both fermentation and cellular respiration
NAD+
The oxidizing agent that accepts electrons during glycolysis
Regenerated from NADH by transferring electrons to the electron transport chain under aerobic conditions
Anaerobic conditions require fermentation to regenerate this
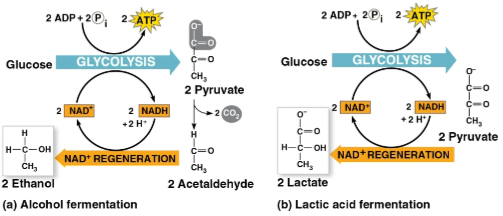
Fermentation
An extension of glycolysis that oxidizes NADH by transferring electrons to pyruvate or its derivatives, includes:
Alcohol fermentation
Lactic acid fermentation
Differs from cellular respiration as electrons are not transferred to the electron transport chain and does not produce nearly as much ATP
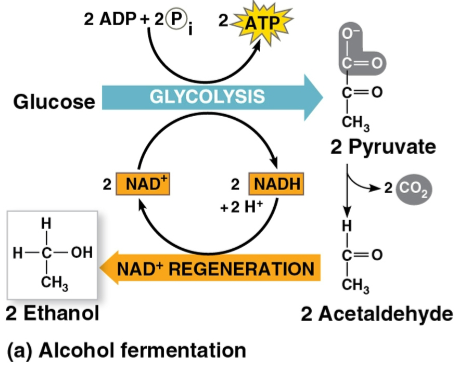
Alcohol fermentation
The conversion of pyruvate to ethanol by:
Releasing CO2 from pyruvate
Producing NAD+ and ethanol
Used in brewing, winemaking, and baking
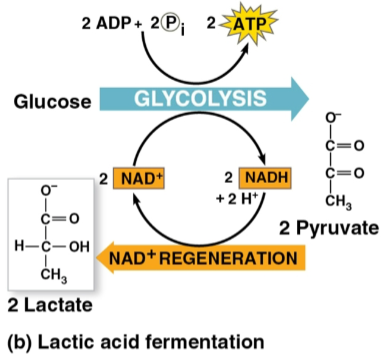
Lactic acid fermentation
The reduction of pyruvate directly by NADH to form lactate and NAD+
Does not release CO2
Used to make cheese and yogurt with fungi and bacteria
Lactate
A substance that was thought to only have been produced by human muscle cells with a lack of oxygen
Actually is produced even under aerobic conditions, thus disqualifying it from being classified as fermentation
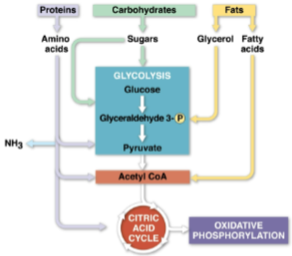
Catabolic pathways
Chains of catabolic reactions that funnel electrons from many kinds of organic molecules into cellular respiration
Seen in glycolysis’ use of many carbohydrates
Deamination
The digestion of proteins used for fuel by breaking them down into their amino acid groups
Nitrogenous waste is excreted as ammonia (NH3), urea, or other products
Nitrogenous waste
Produced as proteins are digested to amino acid groups in the forms of ammonia (NH3), urea, or other products
Glycerol
The substance fats are digested to for glycolysis, broken down in a process known as beta oxidation that produces twice as much ATP as the same mass of carbohydrates
Anabolic pathways
Chains of anabolic reactions to build macromolecules from small molecules in food or from cellular respiration
Seen in protein synthesis from amino acid
Feedback inhibition
The most common mechanism for metabolic control that dictates the level of energy production based on need
Higher ATP demands leads to more respiration
Controlls catabolism by regulating enzymatic activity at strategic points in the pathway