C3 Chemical Changes
1/37
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
acids in a solution are sources of
hydrogen ions
alkalis in a solution are sources of
hydroxide ions
strong acids
completely dissociate into ions in solution
e.g hydrochloric acid is a strong acid. It completely disassociates to form hydrogen ions and chloride ions
HCl → H+ + Cl-
weak acids
only partially dissociate into ions in solution
e.g ethanoic acid is a weak acid. It partially dissociates to form hydrogen ions and ethanoate ions
CH3COOH ⇌ H+ + CH3COO-
base
a substance that reacts with an acid to neutralise it and produce a salt - alkalis ares soluble bases
whats an acid/alkali indicator
indicators show whether a solution is acid, neutral or alkaline
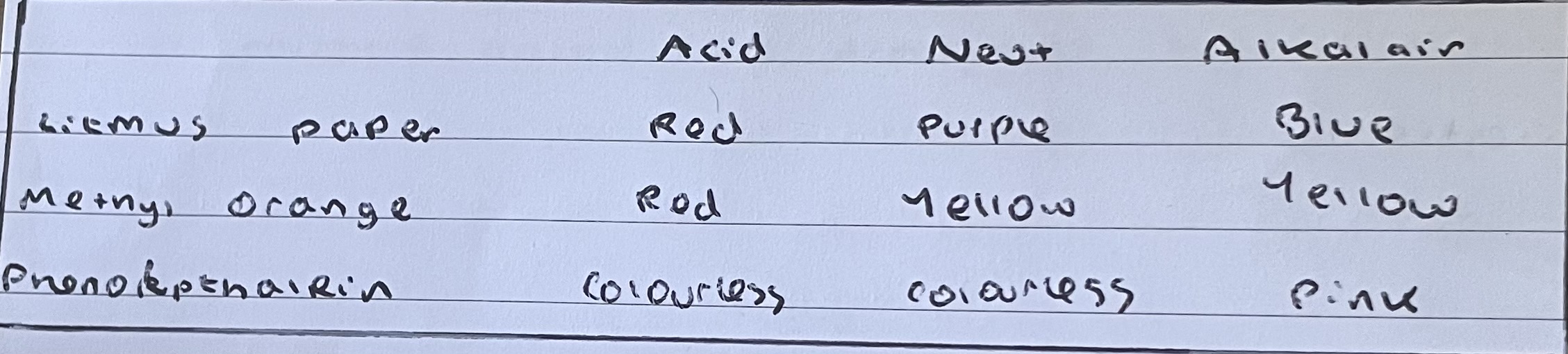
recall the effects of acids and alkalis on indcators including litmus, methyl orange and phenolphthalein
the higher the concentration of hydrogen ions in an acidic solution
the lower the ph
the higher the concentration the hydroxide ions in an alkaline solution
the higher the ph
as a hydrogen ion concentration in a solution increases by a factor of 10
the ph of a solution decreases by 1
neutralisation reaction
neutralisation reaction occurs when an
Acid + Base → Salt + Water
the hydrogen ions (H⁺) from the acid reacts with the hydroxide ions (OH⁻) from the base to form water (H₂O).
Core practical Aim: investigate the change in Ph on adding powdered calcium hydroxide to a fixed volume of dilute hydrochloric acid
dilute
when a solution contains a relatively small amount of dissolved solute
concentrated
when a solution contains a relatively high amount of dissolved solute
What is the general reaction of an aqueous acid with a metal
Acid + Metal → Salt + Hydrogen gas
What is the general reaction of an aqueous acid with a metal oxide
Acid + Metal Oxide → Salt + Water
What is the general reaction of an aqueous acid with a metal hydroxide
Acid + Metal Hydroxide → Salt + Water
What is the general reaction of an aqueous acid with a metal carbonate
Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
chemical test for hydrogen
Collect the gas in a test tube and bring a lit splint near the mouth of the tube. If hydrogen is present, it will burn with a 'squeaky pop' sound.
chemical test for carbon dioxide using limewater
Bubble the gas through limewater (calcium hydroxide solution). If carbon dioxide is present, the limewater will turn milky or cloudy
method for getting a dry sample of an soluble salt (a dry precipitate)
dissolve the solids
mix them together
filter/wash out any impurities
evaporate
method for getting a soluble salt from an acid and an insoluble reactant
dissolve the solids
mix - add the insoluble salt in excess to make sure the solution is completely neutral
filter - removes the excess and any impurities - only salt + water remains
evaporate
method for getting a soluble salt from an acid and a soluble reactant
titrate - to get a precise measurements of the volumes needed for neutralisation
repeat without indicator - done with perfect proportions to prevent excess of either reactant
evaporate - and be left with crystals
Core Practical: Investigate the preparation of pure, dry hydrated copper sulfate crystals starting from copper oxide including the use of a water bath
dissolve
mix
filter
evaporate
general rules of solubility
all common sodium, potassium and ammonium salts are soluble
all nitrates are soluble
common chlorides are soluble except silver and lead
common sulfates are soluble except lead, barium and calcium
common carbonates and hydroxides and insoluble except sodium, potassium and ammonium
what are electrolytes
ionic compounds in the molten state or dissolved in water
describe what electrolysis is
electrical energy from a direct current supply, decomposes electrolytes
describe the movement of ions during electrolysis
positively charged cations migrate to the negatively charged cathode
negatively charged anions migrate to the positively charged anode
oxidisation
loss of electrons (OIL)
reduction
gain of electrons (RIG)
reactivity scale - more reactive than carbon (extracted by electrolysis)
Potassium
Sodium
Calcium
Magnesium
Aluminium
carbon
reactivity scale - less reactive than carbon (extracted by reduction)
zinc
iron
tin
lead
hydrogen
reactivity scale - less reactive than hydrogen (mined)
copper
silver
gold
platinum
electrolysis rule for hydrogen (cathode)
Hydrogen is produced at the cathode if the metal is more reactive than hydrogen
electrolysis rule for halide ions (anode)
oxygen is formed at the anode unless it’s a halide ion (chlorine, bromine, iodine)
Electrolysis of a molten solution steps (Copper chloride)
dissolved measured amount of copper chloride in water
attach inert electrode rods using crocodile clips
put electrodes in copper chloride solution
turn on power supply begin electrolysis
Cu²+ ions will go to the cathode and the Cl- ions will go to the anode
At the Cathode: Copper ions (Cu²⁺) gain electrons and are reduced to copper metal (Cu), which is deposited on the cathode
At the Anode: Chloride ions (Cl⁻) lose electrons and are oxidized to form chlorine gas (Cl₂), which is released at the anode.
half equations
3.27
Core Practical: Investigate the electrolysis of copper sulfate solution with inert electrodes and copper electrodes